ALS News Today Forums › Forums › ALS Progress › Research Topics › RCH-4
Tagged: RCH4
-
ALS victims represent 0.3% of the population. Remarkably, 28% of PALS have a history of going to the gym or similar strenuous activity more than once per week. This is pointed out in the RCH4 website, also confirmed on Utube.
-
Marlon + Ness – – In regards to your figures that: “about 28% of PALS have a history of going to the gym or similar strenuous activity more than once per week.”
According to the Health and Human Services website, “28-34% of adults ages 65-74 are physically active.”
This proves that the percentages are the same for the general population and those who have ALS -
Hello, just a update on my mother she is doing quite well with no side effects what so ever!! The RCH4 charity has been supportive and extremely caring for my mother. Here is her latest monthly score.
<div dir=”ltr”>
</div>
-
It has now been 2.5 years since mum commenced using RCH4 , all provided free of charge by The Charity. Her ALSFRS scores have not declined in this time and she continues to remain stable. Much gratitude and thanks to the charity for their ongoing compassion and support.
Her latest chart.
-
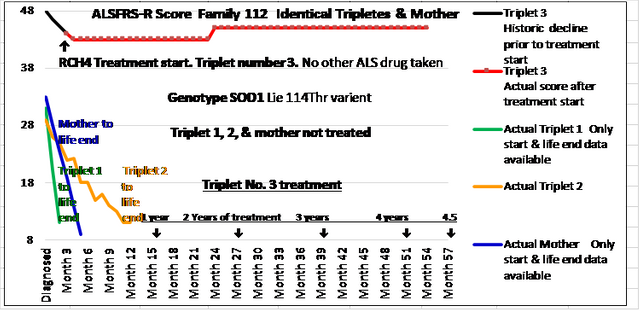
Just wanted to update on my wife who has MND and has been taking RCH4 for 4.5 years now, which has been going really well for her. For those who don’t know my wifes story, she is the only remaining triplet sister who is still alive. She lost her two sisters and her mother, all to this awful disease. They all died withing 18 months of first symptoms! We searched for any kind of treatment that may have a chance of working, as mainstream was offering nothing, and still offers nothing! We dicounted loads of things we could prove didn’t work and costs loads of money. We then came across RCH4 on their website and seemed to offer something slightly different that we though was worth a chance. We applied in Jan 2016 and started receiving in Feb 2016 at absolutely no cost! The rest is history and my wife is still with me. Her ALSFR-S score has frozen at 43 as can be seen on the chart below. If you have any questions on RCH4, let me know and if I can help answer, I will.
Marlon
[img]https://i.ibb.co/QpCdp0P/Nessy-chart-Aug-2020.png[/img]
-
Mum continues to do well on RCH4 , her ALSFRS-R score remains unchanged this month. We are very grateful to the Charity group and hope more Pals have access to RCH4.
-
Hello, just a update on my mom, she is doing quite well on RCH4 it has been 4 years now that she has been taking it. I will try to post her monthly score sheet for everyone to see. We have never been asked for money for RCH4!! This drug could help a lot of PALS out there, but for some reason people in this world don’t want RCH4 to go to market. God only knows, but this drug works to slow the progression of this terrible disease. Sorry I am unable to post mom’s score sheet at this time.
-
We were given the devastating news that mum had MND 3 years ago today 17/11/17. It is one of those moments that is etched in our family’s memories.
We consider ourselves very fortunate however to have found RCH4 and the charity that provides it. Their ongoing care and support is invaluable, Mum remains stable with no real change in her ALSFRS- score. We are so grateful to she is still here with us.
-
A Happy New year to everyone on ALS News Today. I’m very happy to have my wife around for another year. It has been 5 years since my wife’s first symptoms of this awful disease. We thought the worst once we realized what was going on because her two sisters and mother all died within 18 months of onset of MND. Lucky for us we were accepted as recipients of RCH4 (a trial drug) which we then received at no cost to us. It has proved to be a godsend because since starting the drug, her progress has halted.
Shortly after onset, we were having to move her around in a wheelchair for any distance. Here is a video of her walking today which I would say is pretty good for 5 years on.
https://www.youtube.com/watch?v=goobRa-3b7c&feature=youtu.be
Here below is a copy of her ALSFRS-S chart which is produced by the RCH4 suppliers each month. It also shows the disease timelines of her triplet sisters and mother and how short they were 🙁
https://i.ibb.co/BgCXZ6R/Dec2020.png
-
Diana said “The same kind of information, including results from trials, should made readily available from the developers of RCH4. The fact that it is not raises grave doubts in my mind about the product’s efficacy and safety for use.”
Actually Diana, they do. See https://www.als-new-drug.com/rch4-clinical-data
I have been on it now for more than three years and it really helped
-
Hi Dagmar,
I’m having an issue with being able to add mum’s AlSFRS chart.
Can you please provide instructions
Many thanks!
-
Jeanette,
Unfortunately, you cannot add a chart or photos into your comments – – only links. Maybe it would be best to tell us a summary or highlights from the chart? Like if there is improvement, how much and what you observe.
-
-
-
Deleted User
Deleted UserMarch 12, 2021 at 9:26 amResearching RCH4 has me stumped. The “Clinical Data” they publish on RCH4 Clinical data (als-new-drug.com) has a lot of omissions.
1) What was the method of selection for participants during the studies reported on the web site? Were there people turned down or were all applicants accepted? If some were rejected, why? How many?
2) How many subjects were involved, and where is the study they claimed they submitted in 2011 (?). Were there any other studies, and if so, why don’t they publish them on the web site? If they cannot get them published in a professional journal, why not put them out there for the rest of the world to see?
3) Studies are essential in research and development and are based on results from prior work. Each time a study is published others can try to repeat the study to see if the results are valid. If multiple researchers get the same results, it becomes a stepping stone for advancement, and new progress is built on established empirical evidence from past studies.
4) How can the people behind RCH4 justify ignoring the rest of the world, and deny what appears to be an effective treatment for ALS patients ? How many people can they afford to treat, while they deny the rest due to their unwillingness to disclose everything about this product? Without proper research, nobody is going to lend any credibility to RCH4. How many thousand pALS have passed because they have not had an effective treatment like RCH4 available?
The product is injected into muscle, so there has to be a basis for the reason it works, and there is no government that will approve its use without proper studies being conducted, to include trials. These are all points I pulled out of my head as an observer on this issue. I do not dispute that RCH4 does or does not work, only that after this amount of time, the people behind it should have taken affirmative steps to get RCH4 into the mainstream.
-
The RCH4 charity first helped my sister back in 2014 and my mother since 2016. Here is their ALSFR chart:https://i.ibb.co/Cs6Ggdn/zzzzSC.png
To JohnS who asked how they select PALS for treatment and why they have done nothing to move it forward. We are taken on a first come first served basis if / when they have the money. There are no selection criteria. See their website.
From Google, it costs about $41,000 for every patient in a clinical trial. For a small voluntary charity that’s a show stopper. Years ago they offered the whole thing for free as a gift to the ALS Association and the Motor Neurone Disease Association who both refused it.
Hope this helps answer your questions
-
Deleted User
Deleted UserMarch 13, 2021 at 8:29 pmHi Steve,
That did not answer a single question. The representation that the people behind RCH4 are medical professionals with immense experience tells me that they should know what needs to be done to get RCH4 into the mainstream. Studies, reviews, documentation are all lacking from public view. Typically, this information is published in professional journals, but they quit even trying to get that done. Some of the journals will take anything, so its not like they bent over backwards to get there, ya know?
They post all these numbers and figures, and a few people say they have been helped, but thats not going to get the job done. There are ways to do things right, and then wrong, and from what I can see, this was wrong. Publish professional studies on RCH4. If Journals will not take the studies, post them online, for the entire world to see. Answer questions. Open the books to show the world what has been done instead of hide it because that builds mistrust.
I am glad that your relatives, as well as others, have benefitted from RCH4. I just think that it should be available to everybody that wants it, not just a few that some anonymous donor (foundation) can afford. Instead of protecting it and keeping it hidden, put the light on top of the mountain so everyone can see it. If there is nothing to hide, and everything is true, a lot of funding would follow.
-
-
Hi John
You say that studies, reviews, documentation are all lacking from public view. The charity that provides RCH4 for free shows all that stuff on their website. RCH4.org
-
I agree with Jon, but also with Tomasz Boki. Clearly the communication of the RCH4 team could improve.
However I agree that does not mean that RCH4 is not without interest.
I have two remarks:
– Why should we expect that this RCH4 discovery could not be made by another group/ For example look at: doi.org/10.1212/NXI.0000000000000937In conclusion, we observed a decline in TDP-43 reactivity in patients with ALS. The apparent decrease in levels of high-affinity/avidity anti–TDP-43 NAbs correlates with and thereby predicts disease severity. The decline in high-affinity anti–TDP-43 NAbs might impair the capacity to block and neutralize toxic proteins, and although this requires further investigations, data from this study provide rationale for immunotherapy against aggregated TDP-43 as a promising strategy to slow progression of sporadic ALS.
Several companies are working in this area, for example Promis Neuroscience, IMStar, AC immune.
JP Julien (Imstar CTO) had patented (US10202443B2) such antibodies in 2014, well before RCH4 appeared on ALS forums. They didn’t were able to setup clinical trials, probably because clinical trials need a lot of resources out of reach of a small biotech.– What will happen to the pALS who receive RCH4 when the charity will have no funds?
-
-
So the suppliers of RCH4 must be supplying someone data on this drug, as I believe they have just been granted Orphan Drug status which is great news. I would say that if the FDA could scrutineer the drug and believe it has some benefits others might start to take it seriously now and get things moving.
My wife is up to nearly 5.5 years now on RCH4 and is still holding in at an ALSFR-S score of 45 which is just amazing.
-
> as I believe they have just been granted Orphan Drug status
@Marlon+Ness, I just checked the 2020 and 2021 pages of FDA Orphan drug status, and I can’t find any drug which was granted Orphan status for ALS for those two years. But that does not mean anything, for example Spinogenix claims it had been given this status for ALS this year, yet it can’t be found on FDA’s web sitePlease kindly can you give a pointer to a FDA web page?
-
-
Thanks for the link.
In the previous message you wrote:
as I believe they have just been granted Orphan Drug status
Do you agree that this link shows it is NOT approved for Orphan status?
FDA Orphan Approval Status: Not FDA Approved for Orphan Indication
-
-
In 2021 the European drug agency granted Orphan status to Ganglioside GM1 for treatment of ALS. It was presented by 3R Pharma Consulting GmbH, a consultancy organization which represent probably another organization.
As far I know there was no clinical trial about Ganglioside GM1.
-
Jean Pierre, RCH4 does indeed have Orphan Drug Designation (ODD) status. That is a different thing to Marketing Authorisation (MA).
ODD is a major step in getting marketing authorisation.Go to https://www.accessdata.fda.gov/scripts/opdlisting/oopd/
Put in RCH4 and you will be seen that it is certainly designated. -
Marion,
A Orphan Drug Designation only means someone has asked FDA to examine the commercial preparedness of a drug.
Anybody can ask this to the FDA (try it)It was indeed examined, but it was rejected. It is written in the link you posted.
Now, there is a lot of misunderstanding here. The FDA will not give an authorization market to a drug simply because someone has filled in a form on Internet. To have some odd of success, an organization needs to prove to the FDA it can spend $20M on clinical trials and that they have at least several dozen MD/PhDs in their staff to back up their claims. It does not work the other way round.
There are many ongoing scientific work on ALS drugs and that have public information about them.
You can look at JP Julien’s team for serious studies on how to stop ALS. Brian Kaspar (the designer of Zolgensma) is even working on therapies to replace motor neurons! So is Xiang-Dong Fu, in the team of Don Cleveland.
Several companies (including Promis Neuroscience) have designed TDP-43 antibodies and even nanobodies. Other teams have designed CPPs or PROTACs against TDP-43.And if there is a thing that makes me sure one ALS drug will soon get market authorization, it is that TDP-43 aggregates are found also in Alzheimer disease. Alzheimer is not a rare disease.
-
-
-
Jean-Pierre you said some interesting things about the FDA. I have taken the trouble to examine their site.
You wrote-A Orphan Drug Designation only means someone has asked FDA to examine the commercial preparedness of a drug.
It has nothing whatever to do with commercial readiness.
Anybody can ask this to the FDA (try it)
True. Yes, try it yourself Jean-Pierre.
But you at least must have a chemical formula or drug composition, scientific laboratory evidence that it works and an explanation why it does.RCH4 must not only have had laboratory evidence but more importantly, they have years of evidence of safety and efficacy in slowing the progression.
Check it out yourself on the `net
https://rch4.org/rch4-clinical-dataIt was indeed examined, but it was rejected. It is written in the link you posted.
No disrespect but you are wrong. Where does the FDA say it was rejected? They do not.
https://www.accessdata.fda.gov/scripts/opdlisting/oopd/listResult.cfmNow, there is a lot of misunderstanding here.
Yes, that is true. Unfortunately you do not understand a lot of things. Again no disrespect intended.
You confuse orphan designation and marketing approval. Application for marketing approval is public information. I can find no record of the charity ever having applied for marketing approval.The FDA will not give an authorization market to a drug simply because someone has filled in a form on Internet.
Correct. Clinical trials are needed.
To have some odd of success, an organization needs to prove to the FDA it can spend $20M on clinical trials
Untrue. Where do they say $20 million must be proved?
and that they have at least several dozen MD/PhDs in their staff to back up their claims.
At least several dozen PhDs? Again, where do they say that?
There are many ongoing scientific work on ALS dgs and that have public information about them
How many of them have slowed the decline in function? Not one.
Out of the last 80 trials of ALS drugs how many slowed the ALSFRS progression in the PALS population tested? Not one.RCH4 has slowed my mothers decline for more than 3 years without side effects. See her chart above as at last January.
Months now of RCH4 treatment 40
Monthly decline from onset to start of RCH4 -1.29 ALS points
Monthly decline since start of RCH4 -0.075 ALS points
Therefore decline rate slowed by 94%I have no connection with the RCH4 charity but mystified about the reason for all the damaging negativity towards them by people who know everything.
The charity provides support, the only drug that really works and never ask for money.
I mean no disrespect to anyone or their opinions but things are said which are uninformed, unfair and unacceptable thus damaging the interests of other patients.
You may believe that RCH4 is useless despite clear evidence to the contrary, but please do not deprive others from the chance of extending their life. .-
Deleted User
Deleted UserJune 28, 2021 at 1:43 pmHi,
I spent a lot of time over several weeks looking for what’s up with RCH4. I think I can sum it up as the people behind it have not complied with current standards of both testing and submission. I looked at the literature they made available on their site (s) and it is very slim on actual studies. Studies must be submitted to a competent journal, and the journal will proceed to have specialists in the same field review the data prior to publication. The RCH4 submissions never went that far. They never presented complete testing data, development data, or completed submission documentation, to a journal of competent review.
Until they get off the pot and put this together, nobody is going to benefit from RCH4, no matter how effective it is. If the work they have done is real, and I am not saying it is not, but the researchers carry the burden of proof to the right authorities, in a way that it will be accepted. Judging by the costs of RADICAVA (149k per year) it is not about if there is a profitable venture, but the RCH4 development has to be strictly in compliance just like all the other research on the market. There are no shortcuts.
RCH4 almost got there once, and at the very moment that they were given the chance to present all of the data and research, they withdrew it. It was not about money, because there are lots of investors who would willingly finance ventures like this because after it is approved there is a huge profit incentive. So no matter how many people they find, no matter how many they help until they go through the bureaucratic nonsense to get the drug approved it will never make it to market.
It is a shame that they refuse to take these steps because thousand of us die every year that could have been helped.
-
-
<div>Jean-Pierre you are mixing up orphan drug designation with marketing authorisation approval.</div>
<div><span style=”font-size: medium;”>Two completely different things. Designation is a step towards approval. Steve</span></div>-
I agree with you Steve, it’s not the same thing.
Do you have more information about what to understand with this text:
FDA Orphan Approval Status: Not FDA Approved for Orphan Indication
Thanks!
-
-
<div>Hi Jean-Pierre you were wrong about a lot of things but thats OK. Answering your question is simple.</div>
<div>”Approval Status Not FDA Approved” means the drug does not have marketing approval</div>
<div>”Designation Status Designated” means the FDA has awarded it designation as an orphan drug.</div>
<div>I am interested in Jeannete questions. Where does the FDA say $20 million is needed and where do they say that at least a couple of dozen PhD`s are needed.</div>
<div>It is advisable to be careful making statements here and elsewhere. Thanks Jean-Pierre.</div> -
John s
As aretired now academic researchar I can see that you have never been involved in any drug research nor have you ever tried to raise money for it (if not ALSTDI or one of the Associations, it
s impossible). If I am wrong, kindly say how many scientific papers you have published and their references.Complaining about the RCH4 charity who give a lot and ask for nothing is odd. You are hurting other PALS by undermining the future of the charity. You cannot deny that is what you are doing even if you mean well and its unintended.You suggested that the charity get off the pot. You should do the same, stop criticising and ask them for help if you are a PALS.
Thank you
-
Deleted User
Deleted UserJuly 23, 2021 at 11:32 amHello Tomasz,
It really does not make any difference what you retired from. Neither does it matter what and where I come from either. The issue is, that you evaded the entire topic and created a straw man argument. You appeal to authority based on your own experience, yet you did not say a word about the issues I pointed out. That does not establish credibility.
-
-
Results from RCH4 can’t be denied. This chart is from their website http://www.rch4.com
https://i.ibb.co/mJnqqLf/Ness-Als-Netpiccture.png
and below is my wife’s ALSFR’S chart which is amazing considering the terrible fate of the rest of her family.
-
Mum continues to remain stable with RCH4, no change in her ALSFR score.
-
Deleted User
Deleted UserJuly 23, 2021 at 11:33 amI know everyone who is posting on the RCH4 treatment is convinced. My issue is not with them, but it is with the failure of the drug developers that have failed to bring the product to market. It’s not something that should be ignored because if the claims they post are true they should be able to bring it to a scientific journal and get it published. There are dozens of no-cost, open-access journals that publish research. Most of them are peer-reviewed. To claim that they cannot present the required research because they cannot afford it is incredible. There is no reason why they just quit trying and now refuse to find investors to monopolize the treatment and save thousands of lives every year.
Whatever the reason, it should be brought to light instead of hiding it in the dark.
The discussion ‘RCH-4’ is closed to new replies.

