NurOwn Dosing Likely to Continue for ALS Patients in Its EAP
Written by |

Amyotrophic lateral sclerosis (ALS) patients who completed the expanded access protocol (EAP) for NurOwn may soon be eligible for three additional doses of the cell-based therapy, BrainStorm Cell Therapeutics reported.
EAPs, also known as compassionate use programs, are intended to make investigational therapies available outside of a clinical trial to people whose serious or life-threatening conditions have few or no adequate treatments, when the therapy’s benefits are thought to outweigh potential risks.
The company launched an EAP in the U.S. in late 2020, allowing ALS patients who finished the therapy’s pivotal, placebo-controlled Phase 3 trial (NCT03280056) and who met specific eligibility criteria to gain access to NurOwn at no cost.
People with less advanced disease — as measured by the ALS Functional Rating Scale (ALSFRS-R) — were prioritized for the program, based on top-line trial data showing a superior treatment response in those at earlier ALS stages.
These patients received three doses of NurOwn. After reviewing data from the initial EAP, the company announced plans to provide them with three more doses. This means these patients will receive nine NurOwn doses in total, helping to clarify the therapy’s potential long-term benefits.
“Eligible patients now have the opportunity to receive as many as 9 doses of NurOwn in total, allowing additional data collection to better understand the potential benefits of longer-term treatment,” Robert Brown, MD, PhD, one of the Phase 3 trial’s principal investigators, said in a press release.
The initial EAP was designed in partnership with the U.S. Food and Drug Administration (FDA), which also recommended a protocol amendment to enable EAP participants to receive the extra doses.
“We are pleased to be able to provide additional treatments to these patients. This program is an outcome of a fruitful collaboration between the FDA, Patient Advocacy groups and Brainstorm. We look forward to continuing this dialogue with the FDA for the best path forward,” said Chaim Lebovits, Brainstorm’s CEO.
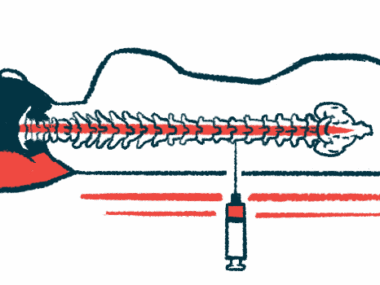
NurOwn is made of mesenchymal stem cells (MSCs) collected directly from a patient’s bone marrow. The MSCs are then expanded and matured to produce high levels of neurotrophic factors, molecules that promote nerve cell growth and survival.
The mature cells are returned to the patient via a spinal canal injection, where they can promote and support nerve cell repair. Of note, using a patient’s own cells minimizes the risk of an immune reaction, as can occur with donor cells.
The Phase 3 clinical trial evaluated NurOwn’s safety and effectiveness in 189 people with rapidly progressing ALS. Patients were randomly assigned to three injections of either NurOwn or a placebo every other month.
Top-line data, supported by recent analyses, showed that a greater proportion of patients on NurOwn responded to treatment (32.6% vs. 27.7%) — as measured by at least a 1.25-point slower rate of decline in the ALSFRS-R, which assesses four functional areas (speech, swallowing and upper and lower extremity movement).
This result failed to reach statistical significance, meaning that the trial’s primary goal was not met. However, pre-specified and post-hoc analyses indicated that people with less severe disease at the study’s start benefited most from NurOwn.
Biomarker analyses also confirmed that NurOwn treatment, compared with placebo, significantly increased the levels of neurotrophic factors in the cerebrospinal fluid (the liquid that surrounds the brain and spinal cord), while biomarkers of neurodegeneration and neuroinflammation decreased.
“This dosing extension for the expanded access protocol is an appropriate next step following the new analysis and biomarkers results of the Phase 3 study. It is deeply appreciated by our ALS patients,” Brown said.
“I applaud BrainStorm for having the conviction to continue their EAP which has brought true hope to those in it. I am also grateful that they recognize the value and importance of EAPs and are willing to invest in doing them right despite being a small company,” said Brian Wallach, an ALS patient and co-founder of I AM ALS.
“I look forward to the day, hopefully very soon, when every person living with ALS has access to NurOwn,” Wallach added.