FAQs about ALS treatment options
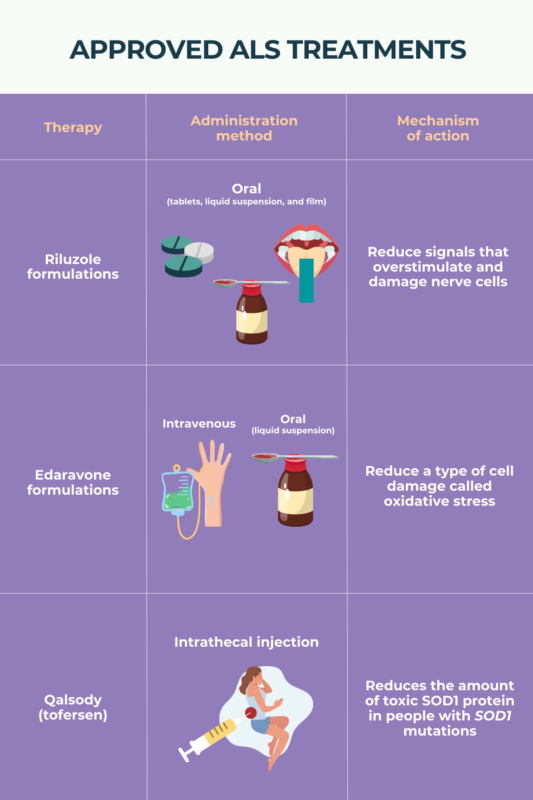
Amyotrophic lateral sclerosis (ALS) is marked by the progressive dysfunction and death of motor neurons, the nerves that control muscle movement. There is no cure for ALS, and no available treatments can reverse motor neuron damage once it has accrued. However, there are several approved ALS treatments that can slow disease progression and extend survival.
Several medications have been approved by the U.S. Food and Drug Administration as amyotrophic lateral sclerosis (ALS) treatments. These therapies have been shown to slow the progression of ALS in clinical trials, but studies have generally not compared them against each other. Furthermore, each patient may respond differently to each therapy; as such, it’s impossible to say whether one medicine or the other is more effective for an individual patient.
There are no stem cell therapies currently approved to treat amyotrophic lateral sclerosis (ALS), although several stem cell-based treatments are in clinical development.
Most insurance providers, including Medicare, will provide coverage for amyotrophic lateral sclerosis (ALS) treatment and care, but specific policies may vary from provider to provider. Patients are encouraged to talk to their clinical team and insurance providers to determine how best to access treatment.
There is no cure for amyotrophic lateral sclerosis (ALS), and no available therapies can reverse damage from the disease. Nonetheless, several approved therapies have been proven to slow the progression of ALS, allowing patients to maintain a higher level of functionality for longer. There also are medications that can be used to help manage specific symptoms of ALS, such as spasticity or excess saliva production.
Related Articles

 Fact-checked by
Fact-checked by