ALS May be Caused by Spreading Protein According to New Study
Scientists at Umeå University have found that superoxide dysmutase (SOD1), a protein that may cause amylotophic lateral sclerosis (ALS) spreads and clumps when injected into mice. The protein deposits also cause ALS-like symptoms. The report, titled “Two superoxide dismutase prion strains transmit amyotrophic lateral sclerosis-like disease” appeared May 3, 2016 in the Journal of Clinical Investigation.
In ALS (also known as Lou Gehrig’s Disease), nerve cells that control movement progressively die in the brain and spinal cord. This causes muscle weakness, paralysis, and eventually loss of breathing and death.
“The occurrence of SOD1 aggregates in nerve cells in ALS patients has been known for a while,” remarked Thomas Brännström, professor of Pathology at Umeå University and the article’s principal author.
“But it has long been unclear what role the SOD1 aggregates play in the disease progression in humans carrying hereditary traits for ALS. We have now been able to show that the SOD1 aggregates start a domino effect that rapidly spreads the disease up through the spinal cord of mice. We suspect that this could be the case for humans as well” he added.
Scientists working at the Departments of Medical Biosciences and Pharmacology and Clinical Neuroscience at Umeå University wanted to know if SOD1 protein clumps (aggregates) are a cause or a consequence of the cell death found in the disease. The investigators actually found two distinct types of SOD1 aggregates in mice. Both variants resulted in SOD1 aggregation spread following a small SOD1 injection into the mouse spinal cord. The aggregates spread, killing neurons as they progressed through the mouse nervous system. The process could mimic the progression of ALS. The animals died after about 100 days following the injection; much sooner than normal mice who had not received the injection.
“The results show that the aggregation of SOD1 plays a critical role in the disease progression — a research hypothesis that ALS researchers in Umeå long has based its work upon,” noted Stefan Marklund, a professor of Clinical Chemistry. “More research is necessary, but our aim is to develop interventions that prevent or stop the fatal course of the disease in carriers of hereditary traits of ALS.”
Now that the scientists have established the ALS model, it may be used to test new treatments that could potentially halt the disease.
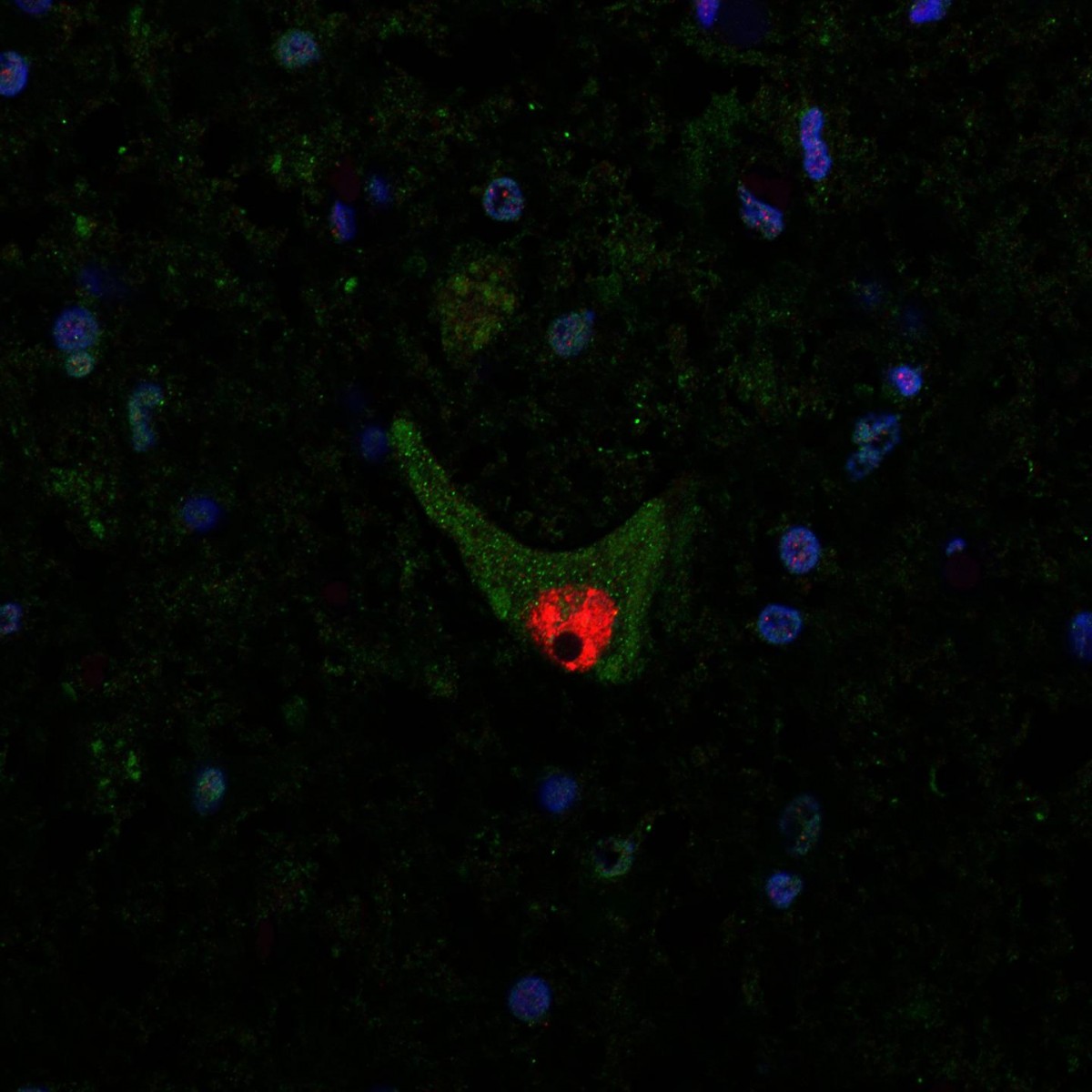
PHOTO CAPTION:
Aggregated SOD1 protein can be seen as green dots in the cell fluid of a motor neuron in the spinal cord of an ALS patient. The red part is the nerve cell nucleus. Credit: Peter Andersen.
PHOTO CREDIT:
Peter Andersen






