Efficacy of riluzole given by intrathecal delivery OK’d for study
Trials will meet American standards, ahead of expected Phase 2 studies
Brain Trust Bio (BTB) has been cleared to launch Phase 1 clinical trials in Australia to test the efficacy of its patented method for delivering therapeutics directly into the spinal canal, with a focus on the amyotrophic lateral sclerosis (ALS) therapy riluzole.
The approach is intended to further enhance riluzole’s therapeutic benefits, which have been associated with slightly extending survival of ALS patients when taken orally.
The trials will be conducted in Australia, but they’ll meet American standards so when its time for Phase 2 trials, the Phase 1 findings will be accepted by U.S. regulators without needing to be repeated, the company said.
“The approval to conduct these trials signifies a pivotal moment in advancing our mission,” Chen Benkler, PhD, co-founder and CEO of BTB, said in a company press release.
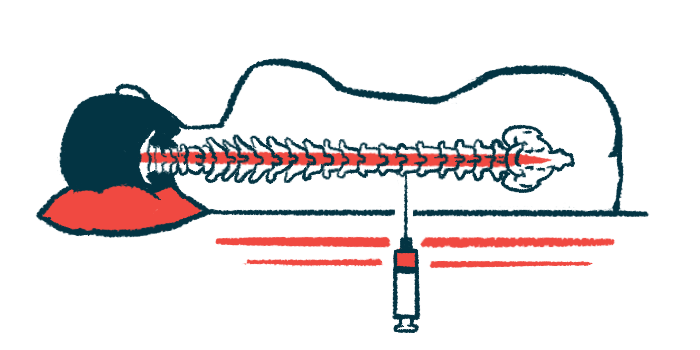
The central nervous system (CNS), that is, the brain and spinal cord, is protected from harmful circulating substances by a tight-knit cellular layer that lines the blood vessels called the blood brain barrier (BBB).
While the BBB protects CNS health, it can pose a challenge for treating neurological diseases. Many medications that are systemically delivered, that is, by swallowing a pill or by being infused into the bloodstream, can’t breach the BBB effectively and therefore can’t reach the tissues where they need to exert their effects.
Benefit of intrathecal delivery in ALS
Intrathecal delivery, where medicine is directly infused into the spinal canal, can get around this problem, because that space is filled with cerebrospinal fluid (CSF), which surrounds the brain and spinal cord. This lets a therapy bypass the BBB and access the CNS, potentially enhancing its therapeutic effects at smaller doses while avoiding off-target side effects elsewhere.
BTB’s patented intrathecal delivery method is designed for continuous infusion of medications. Its early focus is on administering riluzole to ALS patients.
A few versions of riluzole are approved in the U.S. for ALS, but all are delivered systemically. Rilutek is an oral tablet, Tiglutek is a liquid oral suspension, and Exservan is an oral film that dissolves on the tongue.
Riluzole is the only approved ALS therapy known to extend survival, but does so by only about three months, according to BTB. Oral doses can’t be increased to boost its effectiveness because they can cause liver toxicity. The oral medication can also result in cognitive issues and weakness.
BTB believes it can enhance the survival benefits of riluzole without increasing its side effects by administering low daily doses via the intrathecal route. It developed and patented a formulation for this delivery method, which it called IT-riluzole.
The approach has already demonstrated early signs of efficacy, said BTB, with two patients treated for more than two years without any observed side effects.
Preliminary data indicate IT-riluzole may be about five times more effective than existing delivery modes, according to the company. While other methods extend survival by a few months, IT-riluzole may offer another year or more.
The planned Phase 1 trials will involve 10 patients treated with IT-riluzole over six months at two sites in Australia.
The intrathecal approach also lets researchers take CSF samples from patients to look for disease biomarkers.
“Our proposed treatment offers unprecedented access to CSF sampling, enabling us to provide CSF biomarker results to an extent never seen before, further enhancing our understanding and ability to treat CNS diseases,” Benkler said.
The company plans to evaluate the potential of its delivery method in multiple sclerosis and spinal cord cancer, other CNS diseases.







