TDP-43 deficiency leads to changes in physical structures of cell DNA
Study helps explain how TDP-43 abnormalities contribute to nerve cell death
Written by |
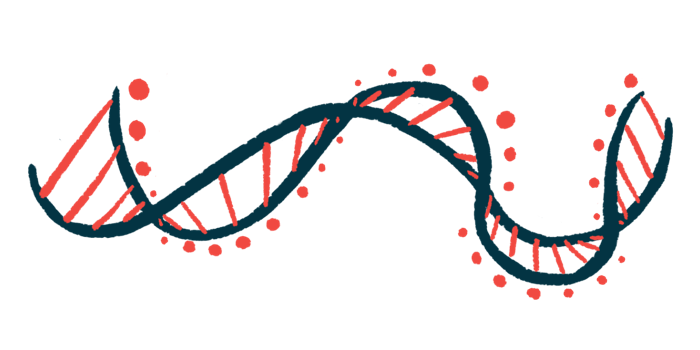
Reduced activity of the TDP-43 protein, a hallmark of amyotrophic lateral sclerosis (ALS), leads to changes in the DNA of nerve cells, which alters the activity of important genes, a new study reports.
These findings may help explain how problems with this protein can contribute to the death of nerve cells in ALS and other disorders marked by TDP-43 abnormalities.
The study, “TDP-43 chronic deficiency leads to dysregulation of transposable elements and gene expression by affecting R-loop and 5hmC crosstalk,” was published in Cell Reports.
In ALS, TDP-43 protein can form clumps outside the cell’s nucleus
Though the causes of ALS aren’t fully understood, abnormalities in TDP-43 are a molecular hallmark of most cases of the disease. TDP-43 normally is present in the nucleus (the cellular compartment that houses DNA), but in ALS and some other neurological diseases, the protein instead forms clumps outside the nucleus.
When TDP-43 forms these clumps, it cannot perform its normal functions. The protein is known to interact with RNA, a molecule made as a temporary intermediate when DNA is “read” to make proteins. However, this new study suggests TDP-43 also alters the shape of DNA, and interferes with molecular modifications to DNA molecules (known as epigenetics).
“Most studies of TDP-43’s function focus on it as an RNA-binding protein. But our analysis really captures how it is playing a genome-wide and epigenetic role, which is not fully appreciated,” Bing Yao, PhD, a co-author of the study and professor at Emory University in Atlanta, said in a university news story.
For these experiments, the scientists used a nerve cell line engineered to produce lower-than-normal amounts of TDP-43. This model “closely corresponds to the neuronal populations exhibiting TDP-43 partial loss of function due to age-dependent pathological sequestration in ALS,” the researchers said.
DNA has a double-helix shape, with two strands spiraling around each other. When a gene is “read,” the two strands of the DNA helix “unzip” to form an opening, and an RNA molecule starts to be put together in the opening. This structure of unzipped DNA and RNA is called an R-loop.
TDP-43-deficient nerve cells can lead to changes in gene expression
Through a battery of molecular tests, the researchers determined TDP-43-deficient nerve cells show widespread dysregulation in where R-loops are located within the genome. The abnormalities in R-loops were closely associated with epigenetic changes, as well as alterations in gene expression (how much certain genes were “turned on or off”), affecting many genes known to be important for nerve cells to maintain their health.
The R-loop abnormalities were also associated with increased activation of transposable elements, which are sections of DNA that are able to jump around to different spots in the genome. Transposable elements aren’t normally active in healthy adult cells. When activated, they can lead to major genetic disruption.
Collectively, these data “provide a common mechanism underlying genome-wide DNA modification dysregulation in several neurodegenerative disorders” in which TDP-43 is not working properly, the researchers wrote. The team noted a need for more studies to work out the detailed molecular mechanisms of exactly how TDP-43 affects R-loops and related genetic elements.
The scientists also think other proteins that can bind to RNA might similarly affect R-loops, which may be a promising area for future studies to explore.
“Our findings suggest that besides TDP-43, other RNA-binding proteins could have this kind of genome-wide role,” Yao said.