Voyager Presents New Data in AAV Gene Therapy for ALS at European Conference
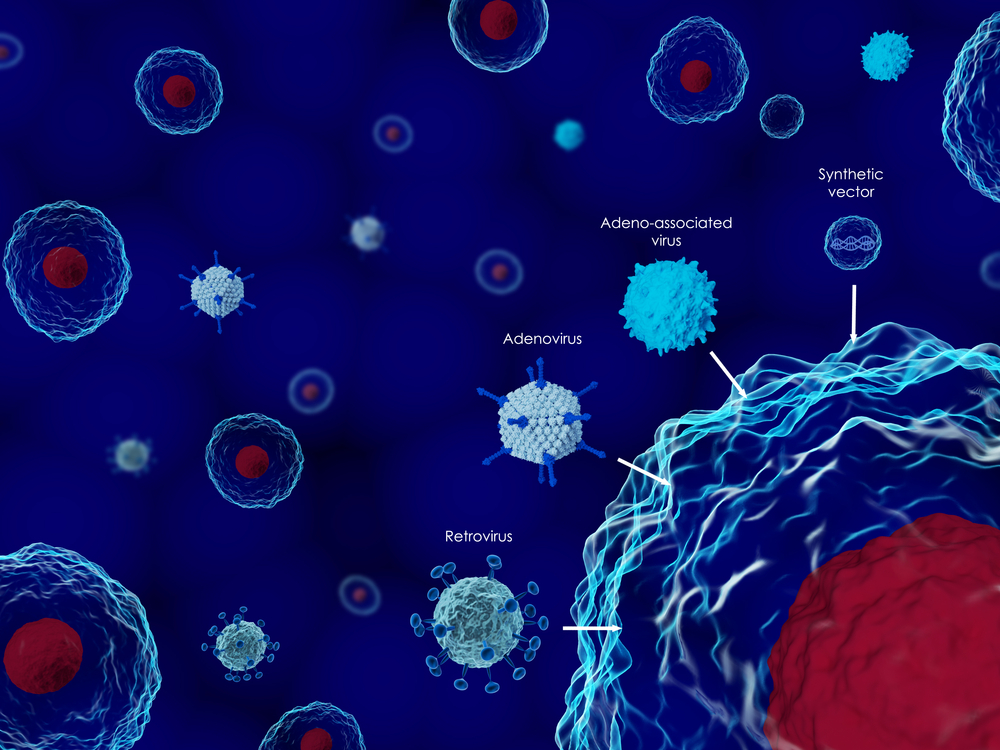
Voyager Therapeutics has recently presented data of its leading adeno-associated virus (AAV) gene therapy for several neurological diseases, including amyotrophic lateral sclerosis (ALS), at the Congress of the European Society of Gene and Cell Therapy (ESGCT), Oct. 17-20 in Berlin, Germany.
In their poster, “Translation of Intrathecal Delivery of an AAV Gene Therapy Targeting SOD1 for the Treatment of ALS,” Voyager researchers presented how their new AAV gene therapy induced a widespread gene transfer to the brain and spinal cord in an animal model of ALS.
Genetic mutations in the superoxide dismutase 1 gene (SOD1) trigger the death of nerve cells and are the leading cause of ALS. Previous studies showed that using AAV as a vector and injecting it directly into the spinal canal of animals with ALS resulted in a successful transfer of genes into the spinal cord.
AAV is a common, naturally occurring virus, which was shown to work as an effective gene therapy delivery vehicle in clinical trials. Advances in AAV vector design proved AAV enabled widespread gene delivery in the brain and spinal cord, making them particularly useful for neurological diseases.
Also, the impact on other tissues outside of the nervous system are reduced, as is the potential of becoming a target to the immune system.
But scientists weren’t sure if AAV gene therapy directed against SOD1 would still show efficacy in a large animal model of ALS.
Voyager researchers used AAV gene therapy carrying a vector that targets SOD1 and delivered it to the spinal canal of a dog with canine degenerative myelopathy, a naturally occurring disease that is similar to some forms of human ALS – including the SOD1 form.
A single injection into the spinal cavity resulted in “74% and 41% suppression, or knock down, of SOD1 mRNA in dorsal root ganglia and spinal cord, respectively,” authors wrote. The therapy was safe and well-tolerated.
These results support the potential future usefulness of AAV gene therapy targeting SOD1 as a treatment for ALS in humans.
“A core competency of Voyager’s gene therapy platform is vector optimization and a critical component of this is optimizing and choosing the capsid, or outer shell of the gene therapy vector,” Dinah Sah, PhD, Voyager’s chief scientific officer, said in a press release.
Voyager’s platform of AAV gene therapy is being developed for several other severe neurological diseases besides ALS, such as Friedreich’s ataxia, advanced Parkinson’s disease, a monogenic form of ALS, Huntington’s disease, and dementia.
“At this year’s ESGCT meeting, we describe exciting progress with novel AAV capsids that enhance the transfer of genes to the brain and spinal cord of non-human primates and in a preclinical model of Friedreich’s ataxia, representing unique opportunities for our current pipeline as well as for future potential programs,” Sah added.