A Caregiver’s ALS Journey of Courage and Support
This is a sponsored post written by Rocío Ibarra in collaboration with TPA
Written by |
This content is sponsored by Tanabe Pharma America, Inc. (TPA) and is intended for US audiences only. Any other present or future content posted by the contributor, not expressly designated as “Tanabe Pharma America, Inc. – sponsored content” is not associated with TPA.
Patient stories reflect the real-life experiences of persons diagnosed with ALS who have been prescribed RADICAVA ORS® (edaravone). However, individual experiences may vary. Patient stories are not necessarily representative of what another person using RADICAVA ORS® may experience.
These experiences are shared by patients and/or caregivers of patients on treatment at the time this content was created.
This is a real-life experience shared by a caregiver of an actual patient who is taking RADICAVA ORS®.
The information provided here is general in nature and is not intended to be a substitute for professional medical advice, diagnosis, or treatment. You are strongly encouraged to seek the advice of your doctor or other qualified healthcare provider with any questions regarding a medical condition.
Individual results may vary. Please see Important Safety Information below, full Prescribing Information, and Patient Information.
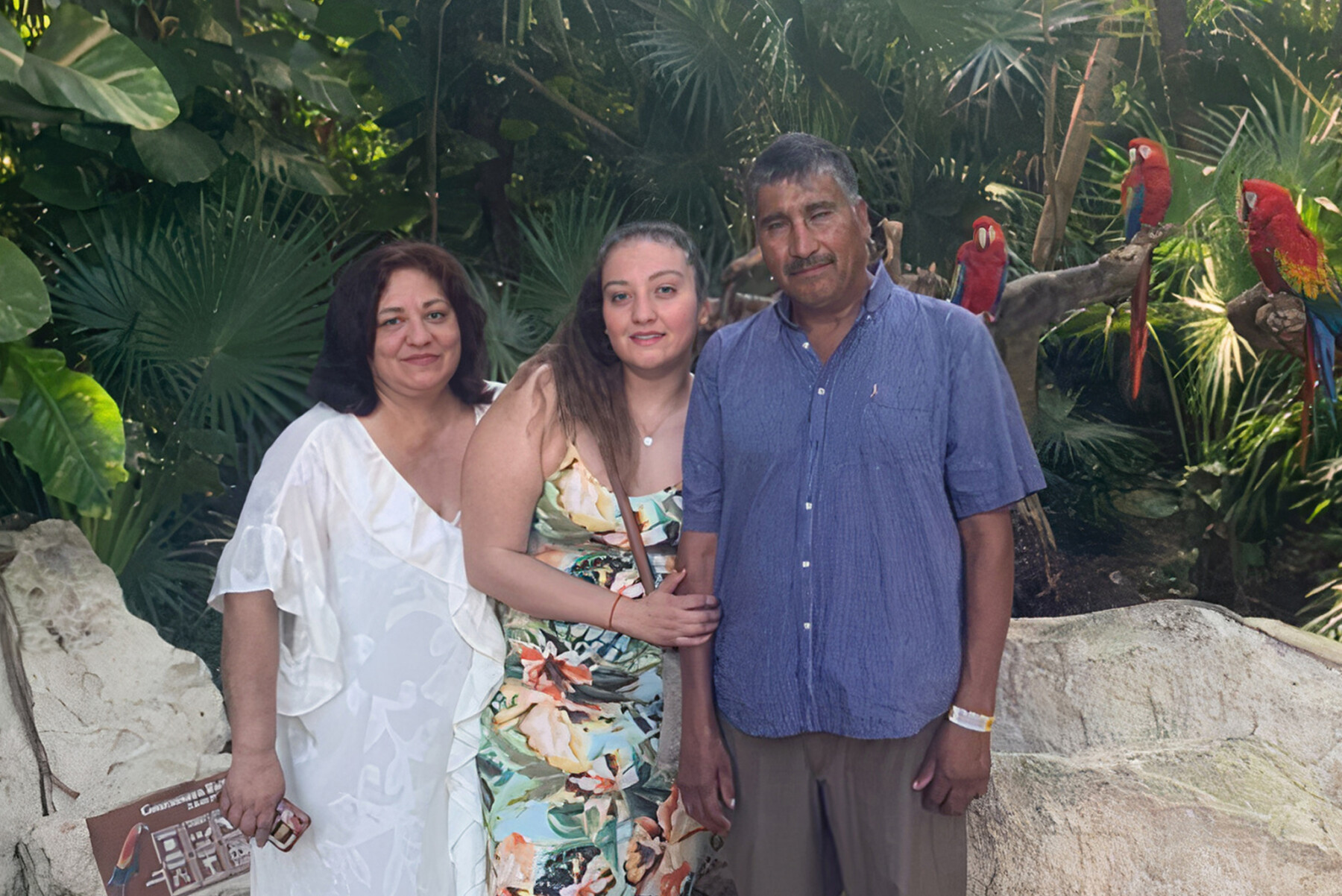
Hearing Francisco’s ALS diagnosis
It was January 30, 2022—the day my husband Francisco and I heard his ALS diagnosis from his neurologist in Mexico. The doctor’s voice was somber as he carefully chose the clearest, least aggressive words to share the news. At the time, I didn’t know what amyotrophic lateral sclerosis (ALS) was, but he used words that reminded me of my neuroanatomy classes, so I knew it was serious.
I tried to hide my concern by using medical terms to talk with the doctor. My husband just looked at me. I tried not to show any emotion, but my face probably showed doubt and disbelief as the doctor went on explaining the diagnosis.
Finally, he gave us a prescription for vitamins and a medication. He said the medication was expensive and would be difficult to locate, but at this point, it was our only option.
Starting Our Life With ALS Together
The drive home from the doctor’s office was silent. I didn’t want Francisco to see I was terrified. I had a long way to go to understand ALS, but I knew we had limited time left. He asked me what the diagnosis meant because he didn’t fully understand yet, but it was easier for me to say that I didn’t understand it either.
Once we got home, I researched ALS online, and my fear increased. Then came the denial. I thought, “This can’t happen to us. We’re going to get another opinion.” So, we saw another specialist in Mexico City. We showed him the test results and discussed the diagnosis, and as we carefully reviewed all the documents, I paid close attention to his face. I was looking for an expression that would give me some relief. But before he gave us his opinion, I knew what he was going to say.
The drive home was completely quiet again. Francisco looked like he was in disbelief. I wanted to hug my husband, but thought it would just make him feel worse.
Facing the Challenge of Finding Treatment
With the weight of the ALS diagnosis on my mind, I later went to a pharmacy in Durango, Mexico, to ask for the medicine the doctor had prescribed. They told me it had to be imported from Germany and would take 15 to 20 days to get. So, we waited several weeks to start the pack of 20 pills. It was very expensive for us, but we hoped it would help slow down the worsening ALS symptoms I was seeing every day. Before he finished the treatment, I requested the next batch of medication.
Since the next round of the medication would be a different dose, the pharmacy said it would take 30 days to receive. They also explained this next batch had to be imported from the US. We were frustrated to once again wait weeks for medication that was supposed to help him. Every day, my anxiety worsened because he wasn’t getting his treatment. Francisco always trusted in my ability to solve problems, and I saw him clinging to me in hopes I’d find some way to help him.
Relying on Family Support to Care for Francisco
At that point, Francisco’s arms were weakening more and more over time. I needed help with his care, so we arranged for our daughter Viviana, who works afternoons, to care for him in the morning. Then I’d take over in the afternoon. We had to restrict Francisco from driving, and this was difficult for him because he felt I was taking away his independence.
He was adamant about riding his bike, which he did for a few days. However, he took a bad fall and hit his face. It was as much a physical blow as it was emotional. This was a very real sign of his declining condition from this disease. He just didn’t have the strength to protect his face.
Living With ALS and Traveling for Treatment
Getting the medicine Francisco needed in Mexico continued to be a challenge. It was even harder to see how ALS was affecting him more and more every day, so we decided to travel to the US with the intention of getting medicine there.
We arrived at the University Medical Center of El Paso, Texas, to see a neurologist specializing in ALS and continue Francisco’s treatment. At that point, Francisco needed an even greater level of care and my daughters and I were working in Mexico. This meant Francisco couldn’t stay alone in Texas for the weeks between treatments. So, we drove 12 hours each way every 15 days from Durango to El Paso for his care.
We made the trips from April to September for medical treatment while trying to get health insurance and disability from his job in the US. It was tough. On some occasions, our daughters helped us with the treatment trips, but eventually I could see his body could no longer withstand those trips.

Making the Major Decision to Move to the US
In October, I retired to take care of Francisco full-time. That’s when we moved to El Paso, Texas. It was life-altering to leave my job, community, family, and grandson. But I knew that if I didn’t make that sacrifice for the care he needed, I would regret it.
In mid-October, he was put on a ventilator. His lungs had deteriorated. I was afraid he could potentially experience respiratory arrest. Shortly after is when he started RADICAVA ORS® (edaravone). Even though he was experiencing weakness throughout his body, we were thankful we could add another treatment to possibly help him slow the loss of physical function he was experiencing.
In the clinical study, RADICAVA® (edaravone) slowed the loss of physical function as measured by the ALS Functional Rating Scale–Revised (ALSFRS-R) by 33% versus placebo. At 24 weeks (about 6 months), patients who did not receive RADICAVA® (66 patients) declined more rapidly in physical function, having lost an average of 2.49 points more than those who received RADICAVA® (68 patients). Common side effects reported include bruising (contusion), problems walking (gait disturbance), headache and fatigue. These are not all the possible side effects of RADICAVA ORS. Talk to your doctor about all the benefits and risks associated with treatment.
RADICAVA ORS® (edaravone) is indicated for the treatment of amyotrophic lateral sclerosis (ALS).
Do not receive RADICAVA ORS® (edaravone) if you are allergic to edaravone or any of the ingredients in RADICAVA ORS.
Please see Important Safety Information below and click here for full Prescribing Information and Patient Information.
Taking a Fall and Moving Forward
This has been a learning experience for me. Throughout our lives, we’ve always had to deal with a series of challenges. I’ve had to take charge and become a person with a strong character, always looking for a way forward.
Even after we moved to the US we had to face challenges. Francisco experienced a second serious fall and we ended up in the hospital. His head needed 7 stitches, and his legs did not recover well. I didn’t know how to encourage him to use a walker. How could I tell him this would not be temporary or say, “You’re not going to recover.” How could I say, “Don’t expect a miracle.” Now, with his strength gone, his hands can hardly move. It’s difficult for him to swallow liquid food and even speak. But we keep persevering. We’re here to help him. He is not alone.
I try to focus on learning more and taking action. I read everything published by the ALS Association. We took part in two studies regarding the costs of the disease and the struggles with medications. We also registered with the National Disease Registry of the United States. Additionally, I’m a member of a Facebook group. It’s a group where Spanish speaking caregivers and patients reunite to talk about their journey with one another. Being able to provide helpful information to others facing the same challenges with ALS gives me purpose.
Standing Together Against ALS
Today is January 23, 2024. Looking back, it’s been one year since the initial diagnosis. We know the disease has progressed, but we can still talk, he can walk, and he can still eat. It doesn’t matter if we do it through difficulty because we still get to be together.
Life presents us with unexpected events that generate uncertainty and fear. Still, now I can say that if we show strength, we can face any situation with the help of the greatest provider of that strength—our family.
I know that the most difficult part is still coming. I just pray that I have the strength not to give up. I know my husband expects a lot from me, and I’ll keep trying to make him smile every day.
Read Francisco’s story for his experience growing up in Mexico, his ALS diagnosis, and his journey with Rocío to receive treatment in the US.
Learn more about ALS and how RADICAVA ORS® may help you.
Learn more about participating in the Share Your Story program at ShareYourALSStory.com.
INDICATION
RADICAVA ORS® (edaravone) is indicated for the treatment of amyotrophic lateral sclerosis (ALS).
IMPORTANT SAFETY INFORMATION
Do not receive RADICAVA ORS® (edaravone) if you are allergic to edaravone or any of the ingredients in RADICAVA ORS.
Before you take RADICAVA ORS, tell your healthcare provider about all of your medical conditions, including if you:
- have asthma.
- are allergic to other medicines.
- are pregnant or plan to become pregnant. It is not known if RADICAVA ORS will harm your unborn baby.
- are breastfeeding or plan to breastfeed. It is not known if RADICAVA ORS passes into your breastmilk. You and your healthcare provider should decide if you will receive RADICAVA ORS or breastfeed.
Tell your healthcare provider about all the medicines you take, including prescription and over-the- counter medicines, vitamins, and herbal supplements.
What are the possible side effects of RADICAVA ORS?
RADICAVA ORS may cause serious side effects, including hypersensitivity (allergic) reactions and sulfite allergic reactions.
- Hypersensitivity reactions have happened in people taking RADICAVA ORS and can happen after your medicine has been taken.
- RADICAVA ORS contains sodium bisulfite, a sulfite that may cause a type of allergic reaction that can be serious and life-threatening. Sodium bisulfite can also cause less severe asthma episodes in certain people. Sulfite sensitivity can happen more often in people who have asthma than in people who do not have asthma.
- Tell your healthcare provider right away or go to the nearest emergency room if you have any of the following symptoms: hives; swelling of the lips, tongue, or face; fainting; breathing problems; wheezing; trouble swallowing; dizziness; itching; or an asthma attack (in people with asthma).
Your healthcare provider will monitor you during treatment to watch for signs and symptoms of all the serious side effects and allergic reactions.
Common side effects reported include bruising (contusion), problems walking (gait disturbance), headache and fatigue. These are not all the possible side effects of RADICAVA ORS.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088. You may also report side effects to www.fda.gov/medwatch or Tanabe Pharma America, Inc. at 1-888-292-0058.
Please see the full Prescribing Information and Patient Information, also available at www.radicavaors.com.
RADICAVA and RADICAVA ORS are registered trademarks of K.K. BCJ-94.
Intended for US Residents Only.
© 2025 Tanabe Pharma America, Inc. All rights reserved.
CP-OE-US-1353 12/25