“Embracing My Now” Part 2: Learning to Live with ALS
Sponsored by Mitsubishi Tanabe Pharma America, Inc. (MTPA)
This content is sponsored by Mitsubishi Tanabe Pharma America, Inc. (MTPA) and is intended for U.S. audiences only. Any other present or future content posted by the contributor, not expressly designated as “Mitsubishi Tanabe Pharma America, Inc. – sponsored content” is not associated with MTPA. Juan Reyes is an actual patient and is being compensated by MTPA for his sponsored posts.
“Oh shoot, I just dropped my coffee, there goes my favorite mug! It was a gift for father’s day…”
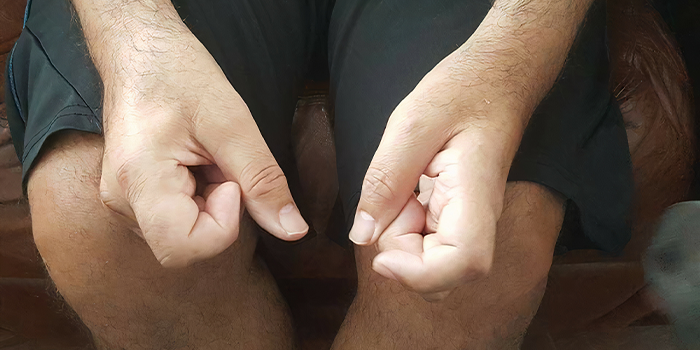
My hands can no longer hold my favorite coffee mugs.
“Hmm, how did I just lose my footing on a perfectly smooth floor? That’s weird, what a klutz…”
“Why are my shoes all scuffed at the toes? I need to go shoe shopping…”
The above are reflections of internal dialogues I had with myself as I began to grow concerned with the intensity and recurrence of these incidents. Ultimately, it was this and noticeable atrophy of my hands that led to seeing my primary doctor.
Initially my doctor wanted to address existing health concerns to ensure my symptoms were not caused by them. Once he ruled these out, my two-year journey to diagnosis began. One of the greatest challenges with ALS is its diverse presentation, varying from person to person. To be honest, when I received the diagnosis, it was a devastating blow, but also a relief. Having a major defining factor such as a name, returns a modicum of control. Allowing, at least our family, to create a plan and to frame our mindset.
Once the gravity of ALS settles in, allowing you to catch your breath, you plan. This plan that encompasses daily living, long-term arrangements and treatment options, is at best fluid. After my diagnosis, we talked about treatment and clinical trials with my doctor.
When it came to RADICAVA® (edaravone), I extensively researched any information available and had lots of questions and in-depth discussions with my neurologist about the benefits and risks of treatment. If you think a treatment including RADICAVA might be for you, talk to your doctor and visit www.radicava.com to learn more. As with any course of treatment, each and every pALS should thoroughly discuss all available options with your doctor.
RADICAVA ORS® (edaravone) and RADICAVA® IV are indicated for the treatment of ALS. RADICAVA ORS® and RADICAVA® IV may cause serious side effects, including hypersensitivity (allergic) reactions and sulfite allergic reactions. The most common side effects include bruising (contusion), problems walking (gait disturbance), and headache. See Important Safety Information below.
Having a good understanding of the effects to any regimen empowers pALS and cALS to have peace of mind. Having peace of mind may allow us to embrace moments that fill our hearts and souls with joy, love and happiness—to “Embrace Your Now.”
Ultimately, what I desire is another cheesy Father’s Day cup, no more stumbles and time to build my shoe collection. Of course, I can’t forget my favorite – silly socks!

My shoes no longer get scuffed since I don’t walk due to ALS.
If you are interested in learning how RADICAVA may help, visit: https://www.radicava.com/patient/understanding-radicava/
Interested in sharing your RADICAVA ORS® or RADICAVA® IV experience with others? MTPA offers the Share Your Story program to allow real people to share real stories. To learn more, and for a chance to share your story, call a JourneyMate Resource Specialist toll free at 1-855-457-6968 or visit https://www.ShareYourALSStory.com.
This information is intended for U.S. audiences only 18 years of age and older. RADICAVA ORS® and RADICAVA® IV are available by prescription only. Talk to your doctor.
IMPORTANT SAFETY INFORMATION
Do not receive RADICAVA (edaravone) or RADICAVA ORS (edaravone) if you are allergic to edaravone or any of the ingredients in RADICAVA and RADICAVA ORS.
Before you take RADICAVA or RADICAVA ORS, tell your healthcare provider about all of your medical conditions, including if you:
- have asthma
- are allergic to other medicines.
- are pregnant or plan to become pregnant. It is not known if RADICAVA or RADICAVA ORS will harm your unborn baby.
- are breastfeeding or plan to breastfeed. It is not known if RADICAVA or RADICAVA ORS passes into your breastmilk. You and your healthcare provider should decide if you will receive RADICAVA or RADICAVA ORS or breastfeed.
Tell your healthcare provider about all the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements.
What are the possible side effects of RADICAVA and RADICAVA ORS?
RADICAVA and RADICAVA ORS may cause serious side effects, including hypersensitivity (allergic) reactions and sulfite allergic reactions.
- Hypersensitivity reactions have happened in people receiving RADICAVA or taking RADICAVA ORS and can happen after your medicine has been given.
- RADICAVA and RADICAVA ORS contain sodium bisulfite, a sulfite that may cause a type of allergic reaction that can be serious and life-threatening. Sodium bisulfite can also cause less severe asthma episodes in certain people. Sulfite sensitivity can happen more often in people who have asthma than in people who do not have asthma.
- Tell your healthcare provider right away or go to the nearest emergency room if you have any of the following symptoms: hives; swelling of the lips, tongue, or face; fainting; breathing problems; wheezing; trouble swallowing; dizziness; itching; or an asthma attack (in people with asthma).
Your healthcare provider will monitor you during treatment to watch for signs and symptoms of all the serious side effects and allergic reactions.
The most common side effects include bruising (contusion), problems walking (gait disturbance), and headache.
These are not all the possible side effects of RADICAVA or RADICAVA ORS. Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088. You may also report side effects to www.fda.gov/medwatch or Mitsubishi Tanabe Pharma America, Inc. at 1-888-292-0058.
INDICATION
RADICAVA and RADICAVA ORS are indicated for the treatment of amyotrophic lateral sclerosis (ALS).
For more information, including full Prescribing Information, please visit www.RADICAVA.com.
CP-RC-US-1991 8/22






