Clinical Trial Defines Next Generation Of Thought-Controlled Neuroprosthetics
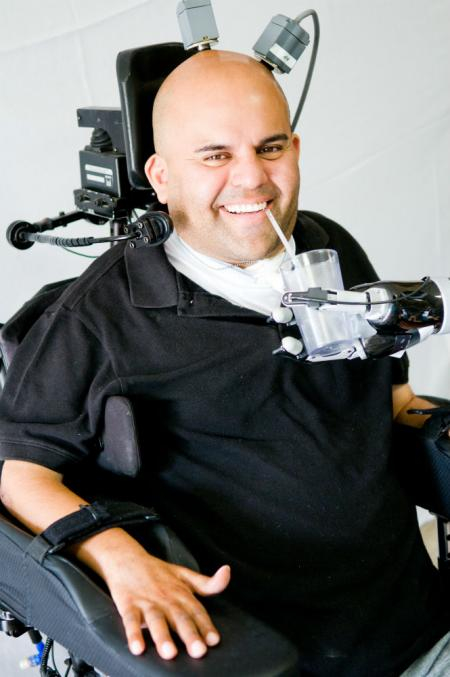
A neural prosthetic device surgically implanted in Erik G. Sorto, 34, left quadriplegic by gunshot wound 13 years ago, has enabled the single father of two to now move a robotic arm via only thought input.
This major breakthrough is the outcome of a clinical collaboration between The California Institute of Technology (Caltech) in Pasadena, California, Keck Medicine of USC at the University of Los Angeles, and Rancho Los Amigos National Rehabilitation Center in Downey, California. Mr. Sorto is the first person ever to have a neural prosthetic device implanted in a region of the brain where intentions are made, giving him the ability to perform a fluid hand-shaking gesture, grip a glass or can of beer, and even play “rock, paper, scissors,” using a separate robotic arm.
The device was implanted at Keck Hospital in April 2013, and since then Mr. Sorto has trained with Caltech researchers and staff at Rancho Los Amigos to control a computer cursor and a robotic arm with his mind. The researchers have been delighted to realize exactly what they were hoping for: intuitive movement of the robotic arm.
Designed to test the safety and effectiveness of this new approach, the clinical trial was led by principal investigator Richard Andersen, the James G. Boswell Professor of Neuroscience at Caltech, Charles Y. Liu, a professor of neurological surgery and neurology at the Keck School of Medicine of USC, and neurologist Mindy Aisen, chief medical officer at Rancho Los Amigos.
 The focus of research at Dr. Richard A. Andersen’s Caltech lab is the neurobiological underpinnings of brain processes, including the senses of sight, hearing, balance and touch, the neural mechanisms of action and the development of neural prosthetics. Dr. Anderson is the recipient of a McKnight Foundation Scholars Award, a Sloan Foundation Fellowship, and the Spencer Award from Columbia University, a McKnight Technical Innovation in Neuroscience Award, and a McKnight Neuroscience Brain Disorders Award. He is also a member of the National Academy of Sciences and the Institute of Medicine.
The focus of research at Dr. Richard A. Andersen’s Caltech lab is the neurobiological underpinnings of brain processes, including the senses of sight, hearing, balance and touch, the neural mechanisms of action and the development of neural prosthetics. Dr. Anderson is the recipient of a McKnight Foundation Scholars Award, a Sloan Foundation Fellowship, and the Spencer Award from Columbia University, a McKnight Technical Innovation in Neuroscience Award, and a McKnight Neuroscience Brain Disorders Award. He is also a member of the National Academy of Sciences and the Institute of Medicine.
 Dr. Liu is a professor of neurosurgery, neurology, and biomedical engineering at the Keck School of Medicine of USC, director of the University of Southern California Neurorestoration Center, and surgical director of the USC Comprehensive Epilepsy Center. In addition, Dr. Liu serves as associate chief medical officer and chair of neurosurgery and spine at Rancho Los Amigos National Rehabilitation Medical Center. Dr. Liu has had a long-standing collaboration with scientists at the California Institute of Technology where he is a visiting associate in the Division of Biology and Biological Engineering.
Dr. Liu is a professor of neurosurgery, neurology, and biomedical engineering at the Keck School of Medicine of USC, director of the University of Southern California Neurorestoration Center, and surgical director of the USC Comprehensive Epilepsy Center. In addition, Dr. Liu serves as associate chief medical officer and chair of neurosurgery and spine at Rancho Los Amigos National Rehabilitation Medical Center. Dr. Liu has had a long-standing collaboration with scientists at the California Institute of Technology where he is a visiting associate in the Division of Biology and Biological Engineering.
 Dr. Aisen’s research and clinical fields include cerebral palsy, advances in neurological rehabilitation, emerging technologies in neuroplasticity, spinal cord injury regeneration, engineering research advances, emerging technologies for persons with disabilities, advances in clinical management of multiple sclerosis and treatment of chronic pain. Dr. Aisen, who led the study’s rehabilitation team, says that advancements in prosthetics like the one implanted in Mr. Sortos hold promise for the future of patient rehabilitation. “We at Rancho are dedicated to advancing rehabilitation through new assistive technologies, such as robotics and brain-machine interfaces. We have created a unique environment that can seamlessly bring together rehabilitation, medicine, and science as exemplified in this study,” she comments in a Caltech releas
Dr. Aisen’s research and clinical fields include cerebral palsy, advances in neurological rehabilitation, emerging technologies in neuroplasticity, spinal cord injury regeneration, engineering research advances, emerging technologies for persons with disabilities, advances in clinical management of multiple sclerosis and treatment of chronic pain. Dr. Aisen, who led the study’s rehabilitation team, says that advancements in prosthetics like the one implanted in Mr. Sortos hold promise for the future of patient rehabilitation. “We at Rancho are dedicated to advancing rehabilitation through new assistive technologies, such as robotics and brain-machine interfaces. We have created a unique environment that can seamlessly bring together rehabilitation, medicine, and science as exemplified in this study,” she comments in a Caltech releas
It is hoped that neural prosthetic devices implanted in the brain’s movement center, the motor cortex, will eventually allow patients with amputations or paralysis to control movement of a robotic limbone that can be either connected to or separate from the patient’s own limb. Neural prosthetic devices have previously been implanted in the brains movement center, the motor cortex, allowing patients with paralysis to control the movement of a robotic limb. The motion, however, is delayed and jerky, in contrast to the smooth and seemingly automatic gestures associated with natural movement. However, by implanting neuroprosthetics in a part of the brain that controls not movement directly but rather the individual’s intent to move, Caltech researchers have developed a new approach to producing more natural and fluid motions.
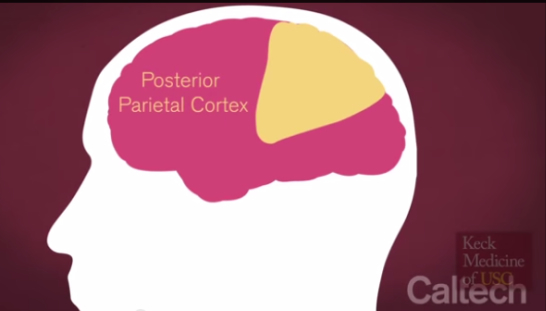
The surgeons at Keck Medicine of USC performed the unprecedented neuroprosthetic implant in a five-hour surgery on April 17, 2013, placing a pair of small electrode arrays in two parts of Mr. Sorto’s posterior parietal cortex (PPC — the portion of the brain’s parietal neocortex posterior to the primary somatosensory cortex) plays an important role in producing planned movements) — one that controls reach and another that controls grasp. Each 4-by-4 millimeter array contains 96 active electrodes that, in turn, each record the activity of single neurons in the PPC. The arrays are connected by a cable to a system of computers that process the signals, to decode the brains intent and control output devices, such as a computer cursor and a robotic arm.
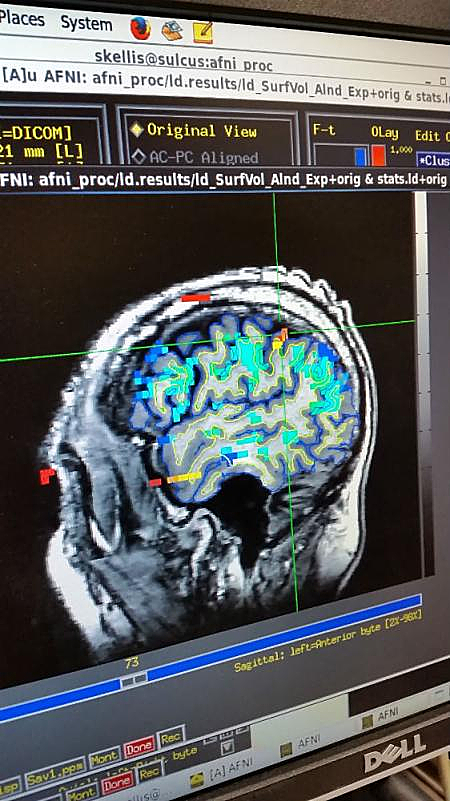
Photo Caption: Example of an fMRI scan used for targeting the device implantation location – Image Credit: Caltech
The arrays were connected by a cable to a system of computers that processed the signals, decoded the intent of the subject, and controlled output devices that included a computer cursor and a robotic arm developed by collaborators at Johns Hopkins University in Baltimore, Maryland.
“These arrays are very small so their placement has to be exceptionally precise, and it took a tremendous amount of planning, working with the Caltech team to make sure we got it right,” says Dr. Liu, who also is director of the USC Neurorestoration Center and associate chief medical officer at Rancho Los Amigos. “Because it was the first time anyone had implanted this part of the human brain, everything about the surgery was different: the location, the positioning and how you manage the hardware. Keep in mind that what were able to dothe ability to record the brains signals and decode them to eventually move the robotic armis critically dependent on the functionality of these arrays, which is determined largely at the time of surgery.”
The implanted device and signal processors used in the Caltech-led clinical trial were the NeuroPort Array and NeuroPort Bio-potential Signal Processors developed by Blackrock Microsystems in Salt Lake City, Utah. The robotic arm used in the trial was the Modular Prosthetic Limb, developed at the Applied Physics Laboratory at Johns Hopkins. Mr. Sorto was recruited to the trial by collaborators at Rancho Los Amigos National Rehabilitation Center and at Keck Medicine of USC. This trial was funded by National Institutes of Health, the Boswell Foundation, the Department of Defense, and the USC Neurorestoration Center.
 “We are at a point in human research where we are making huge strides in overcoming a lot of neurologic disease,” says neurologist Christianne Heck, associate professor of neurology at the Keck School of Medicine of USC and co-director of the USC Neurorestoration Center. ‘These very important early clinical trials could provide hope for patients with all sorts of neurologic problems that involve paralysis such as stroke, brain injury, ALS and even multiple sclerosis.”
“We are at a point in human research where we are making huge strides in overcoming a lot of neurologic disease,” says neurologist Christianne Heck, associate professor of neurology at the Keck School of Medicine of USC and co-director of the USC Neurorestoration Center. ‘These very important early clinical trials could provide hope for patients with all sorts of neurologic problems that involve paralysis such as stroke, brain injury, ALS and even multiple sclerosis.”
Dr. Heck is Medical Director of the USC Comprehensive Epilepsy Program at the Keck Medical Center in Los Angeles, and serves as Vice Chief of Staff at Keck Hospitals of USC and as Co-Director for the USC Neurorestoration Center. She is board certified in neurology with subspecialty training in epilepsy. She is a member of the professional advisor to the Epilepsy Foundation, and has served on the Board of Directors of the National Association of Epilepsy Centers (NAECs). The USC Neurorestoration Center’s primary mission is to leverage partnerships to create unique opportunities to translate scientific discoveries into effective therapies.
“When you move your arm, you really don’t think about which muscles to activate and the details of the movement such as lift the arm, extend the arm, grasp the cup, close the hand around the cup, and so on. Instead, you think about the goal of the movement. For example, ‘I want to pick up that cup of water,'” notes Caltech’s Dr. Richard Anderson. “So in this trial, we were successfully able to decode these actual intents, by asking the subject to simply imagine the movement as a whole, rather than breaking it down into myriad components.”
“We are using our basic science findings to develop a cognitive-based neural prosthesis for paralyzed patients,” Dr. Anderson explains. “This prosthetic system is designed to record the electrical activity of nerve cells in the posterior parietal cortex of paralyzed patients, interpret the patients’ intentions from these neural signals using computer algorithms, and convert the ‘decoded’ intentions into electrical control signals to operate external devices such as robot limbs for activities of daily living or a computer for communications. We are also examining how electrical stimulation of somatosensory cortex can assist in the fine dexterous control of a robotic limb. Our lab is beginning efforts to transition this animal research to clinical trials in paralyzed patients.”
Dr. Anderson notes that the process of seeing a person and then shaking his hand begins with a visual signal (for example, recognizing someone you know) that is first processed in the lower visual areas of the cerebral cortex. The signal then moves up to high-level cognitive posterior parietal cortex area where the initial intent to make a movement is formed. These intentions are then transmitted to the motor cortex, through the spinal cord, and on to the arms and legs where the movement is executed.
High spinal cord injuries can cause quadriplegia in some patients because movement signals cannot get from the brain to the arms and legs. As a solution, earlier neuroprosthetic implants used tiny electrodes to detect and record movement signals at their last stop before reaching the spinal cord: the motor cortex.
The recorded signal is then carried via wire bundles from the patient’s brain to a computer, where it is translated into an instruction for a robotic limb. However, because the motor cortex normally controls many muscles, the signals tend to be detailed and specific. The Caltech group wanted to determine if the simpler intent of handshaking could be used to control the prosthetic limb, instead of asking the subject to concentrate on each component of the handshake — a more painstaking and less natural approach.
Dr. Andersen and his colleagues wanted to enhance the versatility of movement that a neuroprosthetic can offer by recording signals from a different brain region — the PPC. “The PPC is earlier in the pathway, so signals there are more related to movement planning — what you actually intend to do — rather than the details of the movement execution,” he says. “We hoped that the signals from the PPC would be easier for the patients to use, ultimately making the movement process more intuitive. Our future studies will investigate ways to combine the detailed motor cortex signals with more cognitive PPC signals to take advantage of each area’s specializations.”
“For me, the most exciting moment of the trial was when the participant first moved the robotic limb with his thoughts. He had been paralyzed for over 10 years, and this was the first time since his injury that he could move a limb and reach out to someone. It was a thrilling moment for all of us,” Dr. Andersen says. “It was a big surprise that the patient was able to control the limb on day one the very first day he tried,” he adds. “This attests to how intuitive the control is when using PPC activity.”
Mr. Sorto, also naturally thrilled with the quick results, notes in the Caltech release: “I was surprised at how easy it was. I remember just having this out-of-body experience, and I wanted to just run around and high-five everybody.”
“We learned that if he thought, ‘I should move my hand over toward to the object in a certain way’ — trying to control the limb — that didn’t work,” Dr. Andersen says. “The thought actually needed to be more cognitive. But if he just thought, ‘I want to grasp the object,’ it was much easier. And that is exactly what we would expect from this area of the brain.”
“This better understanding of the PPC will help the researchers improve neuroprosthetic devices of the future,” Dr. Andersen says. “What we have here is a unique window into the workings of a complex high-level brain area as we work collaboratively with our subject to perfect his skill in controlling external devices.”
Results of the trial were published in a paper in the May 22 edition of the Journal Science, entitled, “Decoding Motor Imagery from the Posterior Parietal Cortex of a Tetraplegic Human.” (Science, 348 (6237). pp. 906-910. ISSN 0036-8075. https://resolver.caltech.edu/CaltechAUTHORS:20150217-102913989). The paper is coauthored by Afllalo, Tyson and Kellis, Spencer and Klaes, Christian and Lee, Brian and Shi, Ying and Pejsa, Kelsie and Shanfield, Kathleen and Hayes-Jackson, Stephanie and Aisen, Mindy and Heck, Christi and Liu, Charles and Andersen, Richard A. (2015).
The coauthors note that nonhuman primate and human studies have suggested that populations of neurons in the PPC may represent high-level aspects of action planning that can be employed to control external devices as part of a brain-machine interface. However, they observe that there is no direct neuron-recording evidence that human PPC is involved in action planning, and the suitability of these signals for neuroprosthetic control has not been tested.
In the trial, the researchers recorded neural population activity with arrays of microelectrodes implanted in the PPC of the tetraplegic subject. Motor imagery could be decoded from these neural populations, including imagined goals, trajectories, and types of movement, leading the scientists to conclude that these findings indicate that the human PPC represents high-level, cognitive aspects of action, and that it can be a rich source for cognitive control signals for neural prosthetics that assist paralyzed patients.
“The primary mission of the USC Neurorestoration Center is to take advantage of resources from our clinical programs to create unique opportunities to translate scientific discoveries, such as those of the Andersen Lab at Caltech, to human patients, ultimately turning transformative discoveries into effective therapies,” says center director Dr. Charles Liu, who led the surgical implant procedure and the USC/Rancho Los Amigos team in the collaboration. “In taking care of patients with neurological injuries and diseases — and knowing the significant limitations of current treatment strategies — it is clear that completely new approaches are necessary to restore function to paralyzed patients. Direct brain control of robots and computers has the potential to dramatically change the lives of many people.”
Dr. Mindy Aisen, who led the study’s rehabilitation team at Rancho Los Amigos, says that advancements in prosthetics like these hold promise for the future of patient rehabilitation. “We at Rancho are dedicated to advancing rehabilitation through new assistive technologies, such as robotics and brain-machine interfaces,” she notes. “We have created a unique environment that can seamlessly bring together rehabilitation, medicine, and science as exemplified in this study.”
“The reason we are developing these devices is that normally a quadriplegic patient couldn’t, say, pick up a glass of water to sip it, or feed themselves. They can’t even do anything if their nose itches. Seemingly trivial things like this are very frustrating for the patients,” Dr. Andersen concludes. “This trial is an important step toward improving their quality of life.”
Mr. Sorto has signed on to continue working on the project for a third year, and says the study has inspired him to continue his education and pursue a masters’ degree in social work.
Anderson Caltech lab members contributing to the trial were Tyson Aflalo, Spencer Kellis, Christian Klaes, Brian Lee, Ying Shi and Kelsie Pejsa. Keck Medicine of USC team members include Brian Lee, Christianne Heck, Sandra Oviedo, Paul Kim, and Meng Law. Caltech Andersen lab members include Tyson Aflalo, Spencer Kellis, Christian Klaes, Brian Lee, Ying Shi, and Kelsie Pejsa. Rancho Los Amigos rehabilitation team members include Kathleen Shanfield, Stephanie Hayes-Jackson, and Barbara Phillips.
The trial was funded by the National Institutes of Health (grants EY013337, EY015545, P50MH942581A), the Boswell Foundation, the Department of Defense (contract N66001-10-4056) and the USC Neurorestoration Center.
Sources:
The California Institute of Technology (Caltech)
Keck Medicine of USC
Rancho Los Amigos National Rehabilitation Center
Science
Image Credits:
The California Institute of Technology (Caltech)
Keck Medicine of USC
Rancho Los Amigos National Rehabilitation Center