Scientists Gain Insight into Quality Control Mechanism for ALS Faulty Protein Production
Researchers have gained new insight into a cell’s mechanism to get rid of faulty molecules that would otherwise contribute to the development of several neurological diseases, including amyotrophic lateral sclerosis (ALS).
The discovery of this “quality control” mechanism may help in the development of new therapies to prevent neuronal damage resulting from the accumulation of faulty proteins.
The study describing these findings, “Structural Insights Into Ribosomal Rescue By Dom34 And Hbs1 At Near-Atomic Resolution,” was published in the journal Nature Communications.
Human DNA contains the information for the production of proteins necessary for cells’ normal activity stored in the genes. When genes are “read,” a copy of this information is made in the form of an mRNA molecule, which then travels from the nucleus to other cell parts to participate in the production of the correspondent protein.
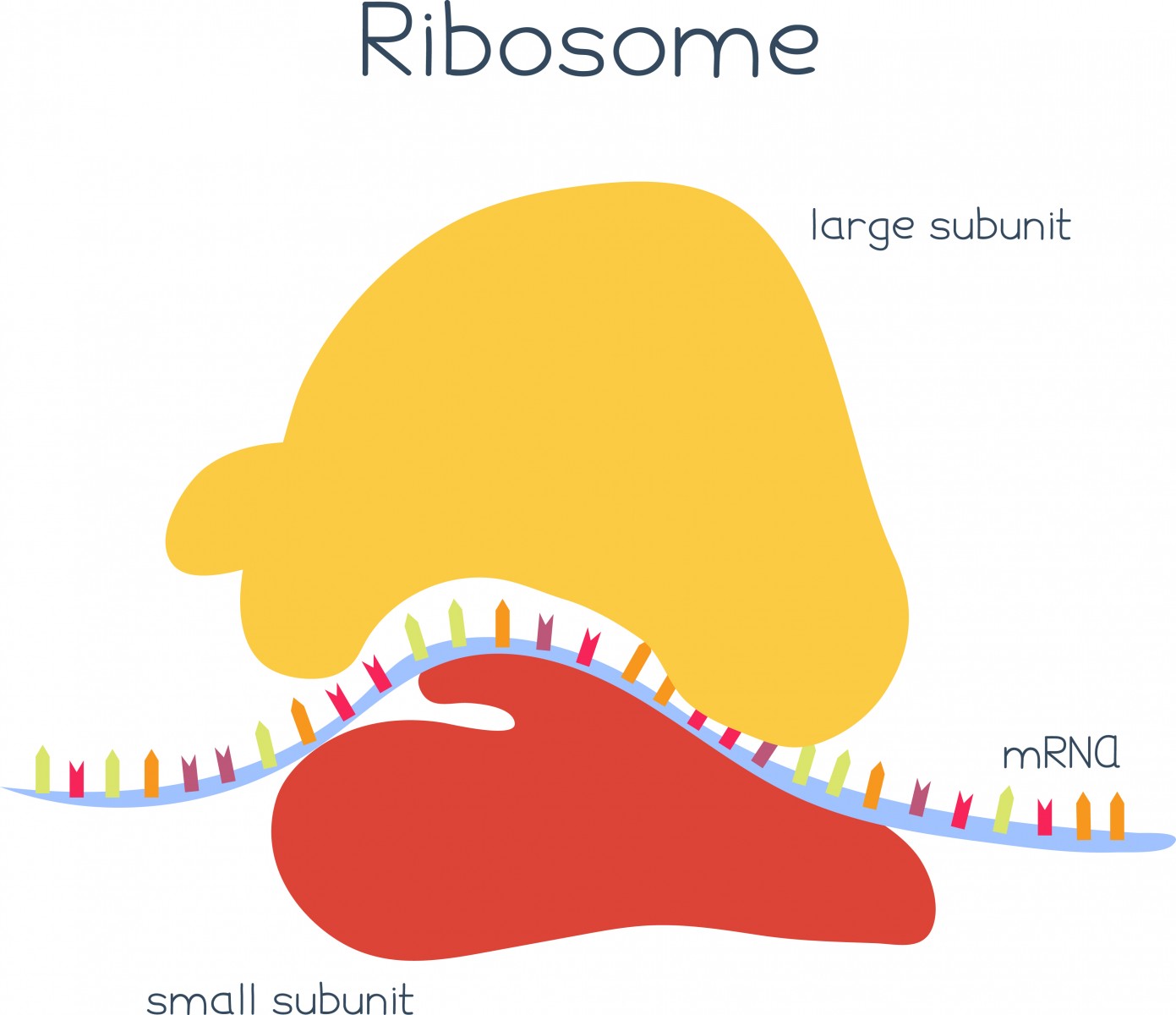
The mRNA molecules are then recognized by ribosomes, small “machines” that will read this information and help produce a protein by identifying small sequences that indicate where the protein should start and stop. This process is called translation, and its surveillance is crucial for each cell to function well.
The translation of faulty mRNAs blocks the work of the ribosomes, impairing protein production and leading to the development of several neurological diseases, including ALS. But two proteins, Dom34 and Hbs1, have the ability to recognize arrested ribosomes and delete the faulty mRNA causing the block. Their exact mechanism of action, however, remained elusive.
Using advanced microscopy techniques, researchers studied how Dom34 and Hbs1 work around mRNAs molecules that have no stop signal, which blocks ribosome activity. They observed that Dom34 and Hbs1 detect and bind to specific regions of the ribosomes that would normally be occupied by mRNA molecules – but that are empty when the mRNA lacks a stop signal – and trigger the disassembly of the ribosomes and the destruction of the faulty mRNA. This mechanism ensures that only arrested ribosomes are targeted, without affecting those that are reading normal mRNA molecules and are working properly.
As several neurological diseases have this control and cleaning mechanism inactivated, leading to the production of aberrant proteins or the accumulation of arrested ribosomes and blocked protein production, the discovery of how Dom34 and Hbs1 work offers insight for novel ways to prevent disease development.
“Research into the effects of aberrant mRNAs and the consequences of inadequate degradation is becoming increasingly significant,” Tarek Hilal, first author of the study, said in a news release.
“Aberrant mRNAs have been found to be particularly common in patients with neurodegenerative disorders such as [ALS],” he added. “Gaining an understanding of the relevant cellular control mechanisms on a molecular level may help us to develop new treatment approaches.”






