Phase 1/2a Trial Recruiting ALS Patients to Test AstroRx, Potential New Stem Cell Therapy
The biotech company Kadimastem is recruiting for a Phase 1/2a clinical trial to test the safety and effectiveness of its leading cell therapy, called AstroRx, in patients with amyotrophic lateral sclerosis (ALS), after positive results were seen in animal models of the disease.
The study showed that AstroRx, made of healthy cells called astrocytes that are derived from human embryonic stem cells, delayed disease onset and prolonged survival and muscular activity of mice and rat models of ALS.
The results were published in the journal Stem Cell Research & Therapy in a study titled, “Safety and efficacy of human embryonic stem cell-derived astrocytes following intrathecal transplantation in SOD1G93A and NSG animal models.”
While the loss of motor neurons is the key event in ALS, a growing amount of evidence suggests the malfunction of another group of cells in the central nervous system, called astrocytes, also play a role in ALS worsening.
Astrocytes are star-shaped cells surrounding neurons in the brain and spinal cord. The evidence suggests that astrocytes may contribute to the progression of ALS via different mechanisms: the secretion of toxic proteins or inflammatory molecules that induce the death of motor nerve cells, among other processes.
These findings suggest that transplanting healthy astrocytes could compensate for the loss of function of malfunctioning astrocytes in ALS, researchers said.
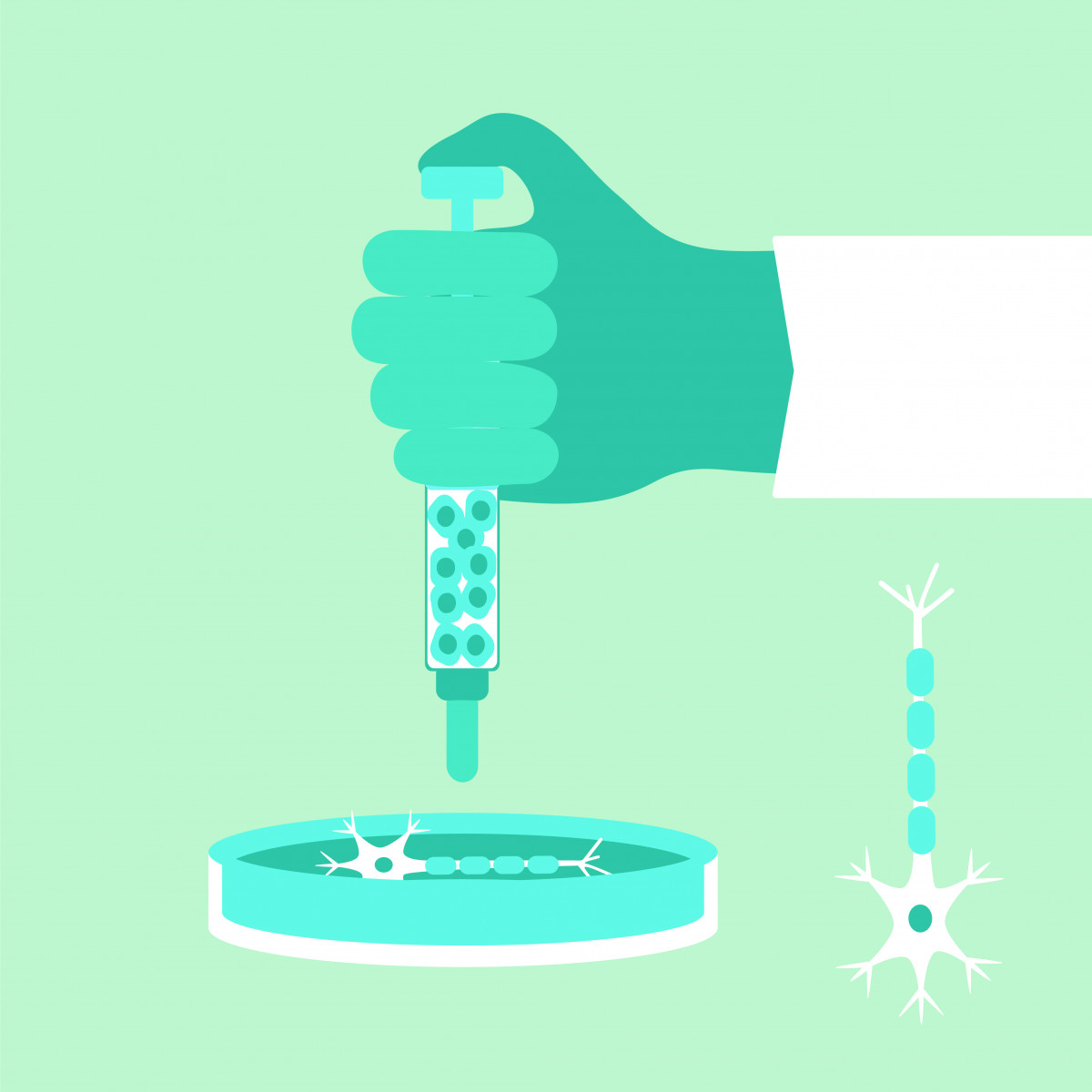
To test their hypothesis, they first developed a protocol to use human embryonic stem cells as a source for producing large quantities of astrocyte progenitor cells (APCs).
APCs can be frozen and stored and, when needed, used to produce healthy populations of astrocytes.
Researchers tested their newly produced population of astrocytes in the lab and found that they reproduce several of the features of healthy astrocytes: They promote the growth of nerve cell axons and protect motor neurons from oxidative stress.
These healthy astrocytes also released a variety of factors with neurogenic – they promote the generation of nerve cells – and neuroprotective activities.
They then moved to animal studies to test the potential therapeutic activity of their healthy astrocytes, which the company calls AstoRx. They injected healthy astrocytes directly into the spinal canal of mice with ALS.
The team tested two different regimens – cells delivered just once or in two loads (each separated by a 30-day period) – and compared the animals’ outcomes to sham-injected mice (the controls).
The results showed that the double transplant of AstroRx significantly delayed disease onset by 119 days, compared to 112 days in sham-injected mice.
Motor performance, assessed by the rotarod test and neurological scoring, was also significantly improved in mice injected twice with healthy astrocytes, and led to a slight increase in mice survival.
They also tested the double transplant method in a rat model of ALS where AstroRx was administered via intrathecal injection by lumbar puncture – an injection administered into the spinal canal – and a route of administration closer to what would be used in a human patient.
In this case, they injected a higher load of cells, divided into two injections. Compared to control rats, transplanting AstroRx led to a significant delay in disease onset, researchers found. In the control rats the disease manifested between 160 and 170 days, while in the transplanted rats, disease onset was closer to 200 days.
Moreover, the decline in the animals’ motor function and loss of forelimb muscle strength was significantly slowed after transplant. Transplanting the cells didn’t result in any tumors.
Overall, “these findings demonstrate the feasibility, safety and potential efficacy of intrathecal injections of hES-AS [healthy astrocytes] for the treatment of ALS,” the study concluded.
The Phase 1/2a clinical trial (NCT03482050) of AstroRx is being conducted at the Hadassah Ein-Kerem Medical Center in Israel. More information about enrollment can be found on the trial’s official web page here.
“In the research, we developed a method which enables the use of stem cells for the production of large quantities of healthy support cells, similar to those in the human brain, and the cells have been proven to possess several mechanisms of action for the protection of neuron survival,” Prof. Michel Revel, Kadimastem’s chief scientist, said in a press release.
“After their injection into the spinal cord fluid, the cells demonstrated therapeutic efficacy in lab animals with ALS,” he added. “Moreover, the treatment was proven safe in a large animal study, which enabled the approval of the clinical trial in ALS patients.”
Yossi Ben-Yossef, Kadimastem’s CEO said the company was proud of its potential new therapy and the study results published in the journal.
“I hope and believe that treatment with AstroRx will improve the condition of the patients participating in the trial,” he added.