Advocates ask for new FDA review of stem cell therapy NurOwn
FDA petitioned to reconsider NurOwn based on available trial, real-world data
A coalition of amyotrophic lateral sclerosis (ALS) patients and their family members has filed a petition to the U.S. Food and Drug Administration (FDA) asking the agency to review again available data for the stem cell therapy NurOwn (debamestrocel).
The petitioners include ALS patients who received NurOwn in clinical trials, as well as those who have been unable to access the medication, according to a press release.
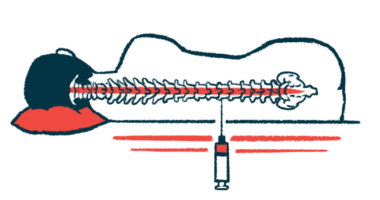
NurOwn works by collecting stem cells from a patient, engineering them in a lab so they’ll secrete molecules that promote nerve cell health, and then injecting the modified cells into the patient’s spinal canal. The goal is to improve the health and survival of the nerve cells that become damaged and die in ALS.
The treatment’s development for ALS has been tumultuous. Its developer, Brainstorm Cell Therapeutics, sponsored a Phase 3 clinical trial (NCT03280056) that tested NurOwn against a placebo in rapidly progressing ALS patients with the aim of showing that the therapy slowed disease progression. The trial had negative results, however, and overall progression rates were comparable among patients given NurOwn or the placebo.
Some benefits of NurOwn seen for patients with less advanced ALS
However, some benefits were seen for patients with less advanced disease. This has led some scientists to argue the trial had showed negative results due to a floor effect — in other words, some patients already had such advanced disease that a slowing in progression couldn’t realistically be measured.
Based on these findings, Brainstorm applied to the FDA seeking approval of NurOwn over the agency’s objections. But when an FDA advisory committee voted that available evidence was not enough to support the efficacy of NurOwn in ALS, the company withdrew its application.
Brainstorm is now working on a new Phase 3b trial called ENDURANCE (NCT06973629) in hopes of proving the therapy’s efficacy. The trial’s protocol has been reviewed by the FDA under a special protocol assessment, and the regulatory agency has confirmed it’s suitable to support a future application for potential approval.
The new petition is asking that the FDA reconsider approving NurOwn based on the clinical trial data as well as new real-world evidence and data from an expanded access program (EAP), which provides access to investigational products outside of clinical trials.
For example, of the 10 patients who participated in the EAP, all of whom had previously completed the seven-month Phase 3 trial, all survived for at least five years without receiving a tracheostomy (a tube to help with breathing). In natural history studies, 20% of ALS patients survive five years after the onset of symptoms.
The petition also highlights experiences from people who have received NurOwn in right-to-try programs, such as Matt Bellina, a Navy pilot with ALS who has blogged about experiencing improvements including fewer issues eating, an easier time breathing, and more mobility.
‘ALS is stealing decades from our lifespans’
The petition argues that, if the totality of the data are considered, then available findings do meet the requirements needed to secure FDA approval — either full approval, or a conditional approval that might allow the therapy to be brought to market while additional testing on its safety and efficacy are carried out. As such, the petition is asking the FDA to reconsider NurOwn.
“ALS is stealing decades from our lifespans,” the petition authors wrote. “Just as the FDA acts with urgency for people with terminal cancer, the Citizens’ Petition asks the FDA to act with the same urgency as ALS is killing our motor neurons. Please don’t let another generation of people with ALS die waiting when we know a stem cell therapy can help us live.”
Brainstorm was not involved in creating or submitting the petition, but the company stated in a press release that it welcomes the FDA’s willingness to reconsider the data.
“We respect the FDA’s independent review process and welcome its consideration of this request,” said Chaim Lebovits, president and CEO of Brainstorm. “We continue to stand firmly behind the scientific integrity of BrainStorm’s data and remain committed to working collaboratively with the FDA and the ALS community to advance the development of safe and effective therapies.”
Mary Kay Turner, senior vice president of advocacy and public affairs at Brainstorm, added: “The ALS community has been a powerful voice in advocating for new approaches to treatment. We stand with advocates in supporting efforts that prioritize both data-driven decision-making and urgency for patients facing this devastating disease.”