Easing the Nerve Cell Traffic Jam May Be a Way to Treat ALS, Research Suggests
A traffic jam inside nerve cells is a feature of most forms of amyotrophic lateral sclerosis and frontotemporal dementia, a study shows.
It suggests that easing the jam is a potential strategy for treating ALS. Therapies to do this are moving into clinical trials, the researchers said.
The study, “TDP-43 pathology disrupts nuclear pore complexes and nucleocytoplasmic transport in ALS/FTD,” was published in the journal Nature Neuroscience.
Mutations of the protein TDP-43 are seen in nearly all cases of ALS and half of FTD cases. While healthy TDP-43 proteins can shuttle between the nucleus of cells and their cytoplasm, or body, mutations of the protein promote the formation of toxic clumps in cytoplasm.
But how cytoplasmic clumping causes neurodegeneration has been poorly understood. To answer this question, researchers at Emory University School of Medicine and the Mayo Clinic in Jacksonville decided to check for other proteins trapped in TDP-43 clumps.
They used a tracking technique called proximity-dependent biotin identification, or BioID. It involves an enzyme called biotin ligase being fused with TDP43, then delivered into cells. Once in a cell, the protein delivers a tag called a biotin to all nearby proteins.
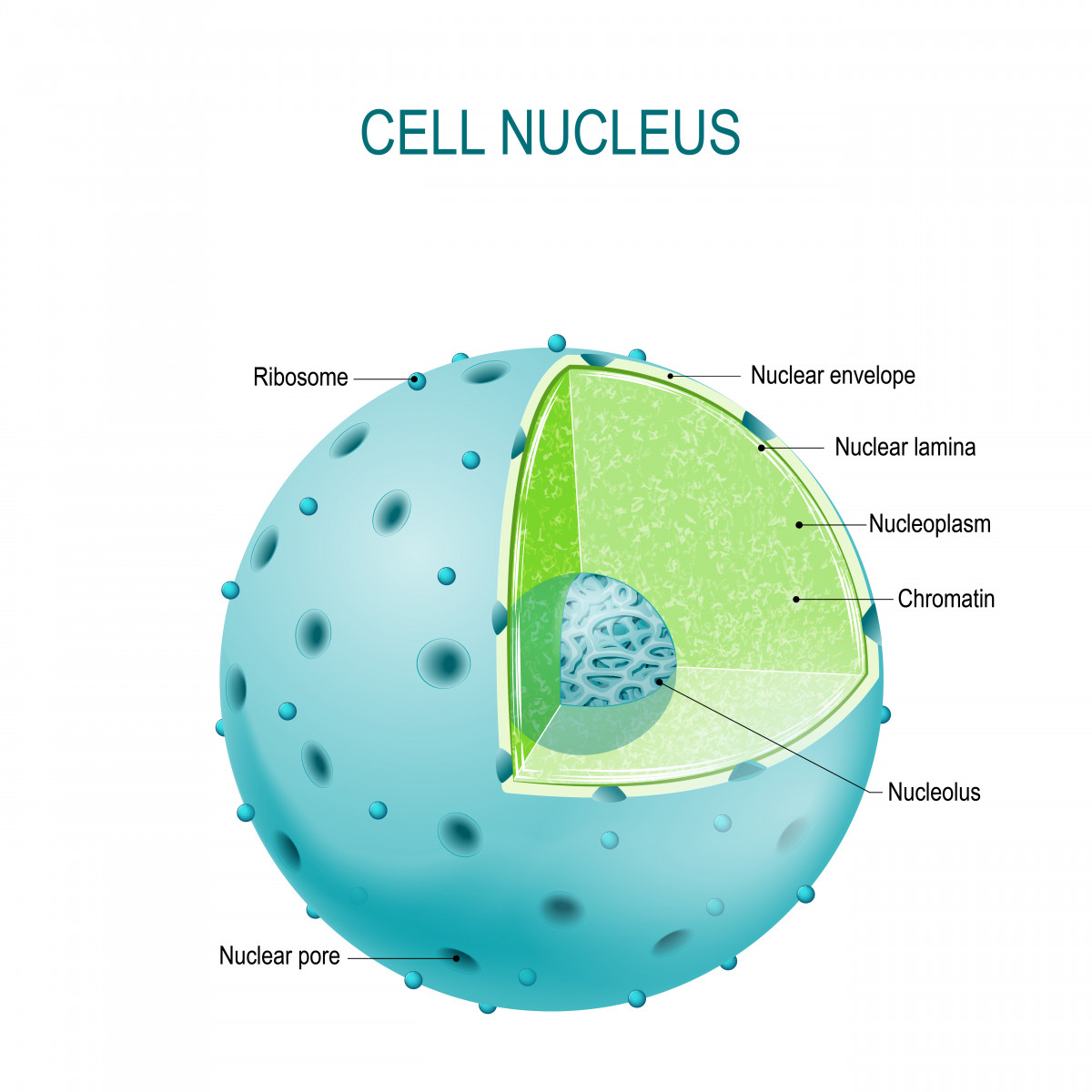
When they examined the nerve cells of ALS patients, researchers found that TDP-43 clumps were full of proteins that belonged to nuclear pore complexes, which regulate traffic in and out of the nucleus.
The clumps trapped the protein, preventing it from going into the membrane of the nucleus and forming a pore. This led to changes in the membrane and pore’s shapes, interfering with the importing of protein to the nucleus and the exporting of RNA molecules to the cytoplasm.
Mayo Clinic researcher Wilfried Rossoll said in a press release that the research showed TDP-43 can cause the traffic jams seen in ALS “and potentially other neurodegenerative diseases with TDP-43 pathology, such as frontotemporal dementia.” He was the study’s lead author.
Researchers detected the changes in skin cells of ALS patients with both inherited and non-inherited forms of the disease, and in the nerve cells of ALS patients with TDP-43 mutations.
They also looked at brain tissue of ALS patients with TDP-43 clumps who had died. Again, they saw clumps of nuclear pore proteins, both in patients with inherited and non-inherited forms of ALS.
“TDP43 pathology is seen in about 98 percent of ALS cases and about 50 percent of FTD cases, and our work supports the now accepted conclusion that nucleopore abnormalities are not restricted to C9orf72 expansion mutations,” Glass said. “Confirming the in vitro data in human brain tissue was very important, making the findings that much more relevant.”
The findings led the researchers to look at whether preventing the nucleus from exporting proteins to the cytoplasm, using a Karyopharm Therapeutics drug called KPT-335 or verdinexor, could prevent nerve cell death. Their rationale was that preventing the exporting would not only compensate for disrupted protein importing but also prevent TDP-43 from entering the cytoplasm and forming clumps.
Verdinexor cut the deaths of clumped TDP-43 nerve cells by more than half.
“These results suggest that suppression of TDP-43 toxicity via pharmacological or molecular inhibition may be a valid strategy for rescuing the defective nucleocytoplasmic transport function,” the researchers wrote.
In fact, compounds similar to KPT-335 have already shown effectiveness in animal models of ALS, and clinical trials of them are under way.
“A better understanding of the role of TDP- 43 in intracellular transport pathways may help in developing therapeutic strategies for ALS/FTD and other TDP-43 proteinopathies,” the researchers concluded.