First patient dosed in a Phase 1 study testing AMX0114 in ALS
LUMINA trial seeks to recruit up to 48 people throughout North America
The first patient has been dosed in a Phase 1 clinical study testing AMX0114, an experimental therapy being developed by Almylyx Pharmaceuticals for amyotrophic lateral sclerosis (ALS). The treatment targets calpain-2, a protein believed to contribute to nerve cell damage in ALS.
Called LUMINA (NCT06665165), the study was cleared to begin in the U.S. after the Food and Drug Administration (FDA) lifted a clinical hold in January, allowing Amylyx to start screening, enrolling, and dosing participants. The trial aims to recruit up to 48 people with ALS at multiple clinical sites throughout North America. Currently, enrollment is ongoing at a site in Ontario.
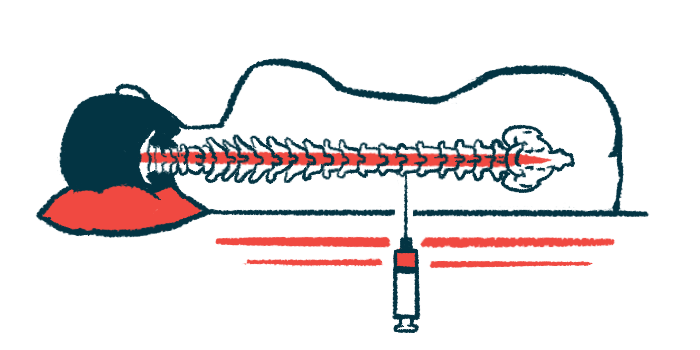
Participants will be randomly assigned to receive either AMX0114 or a placebo via intrathecal injection, that is, into the spinal canal. Each participant will receive up to four doses, given once a month. For every person receiving a placebo, three will receive the therapy, which is being tested across multiple ascending doses. Early results from the first participants are expected this year.
“ALS is a devastating and fatal neurodegenerative disease with limited treatment options, underscoring the urgent need for new therapeutic approaches that target the underlying mechanisms driving ALS progression,” Sabrina Paganoni, MD, PhD, principal investigator of the LUMINA trial, said in a company press release. “Dosing the first participant in LUMINA is a step toward a potential treatment option for people living with ALS and their loved ones.”
What is AMX0114?
AMX0114 is an antisense oligonucleotide (ASO) that’s designed to prevent or slow the degeneration of axons, the long extensions of nerve cells that transmit electrical signals, which is a key driver of ALS progression. It targets calpain-2, an enzyme that’s elevated in ALS and is believed to play a central role in motor neuron damage by promoting axonal degeneration.
The therapy is intended to bind and suppress the messenger RNA, or mRNA, for calpain-2, a template molecule that contains the genetic instructions cells’ machinery reads to build a functional protein. By reducing calpain-2, AMX0114 seeks to protect motor neurons and slow ALS progression.
LUMINA’s main goal is to assess AMX0114’s safety and tolerability by monitoring side effects and any complications. Researchers will also evaluate how the drug behaves in the body, that is, its pharmacokinetics, and how it affects its biological target, called pharmacodynamics.
The study will also track early signs of therapeutic effect by measuring changes in neurofilament light chain (NfL), an established marker of nerve cell injury, and calpain-2 levels in the spinal fluid. The study will also monitor participants’ functional abilities using the ALS Functional Rating Scale-Revised, which assesses daily activities and physical abilities such as speech, movement, and breathing.
In preclinical studies, AMX0114 produced a strong and durable reduction of calpain-2 levels, which was associated with lower NfL levels and improved motor neuron survival in different disease models. Animal studies also showed the treatment was safe and well tolerated.
“AMX0114 targets calpain-2, which has been found to be an important contributor to axonal degeneration and studied over decades of research as a potential target for the treatment of ALS and other neurodegenerative diseases. We are excited to progress AMX0114 into the clinic for people with ALS as we work to advance a potential therapy for this relentlessly progressive, fatal disease,” said Camille L Bedrosian, MD, Amylyx’s chief medical officer.