FAQs about Rilutek
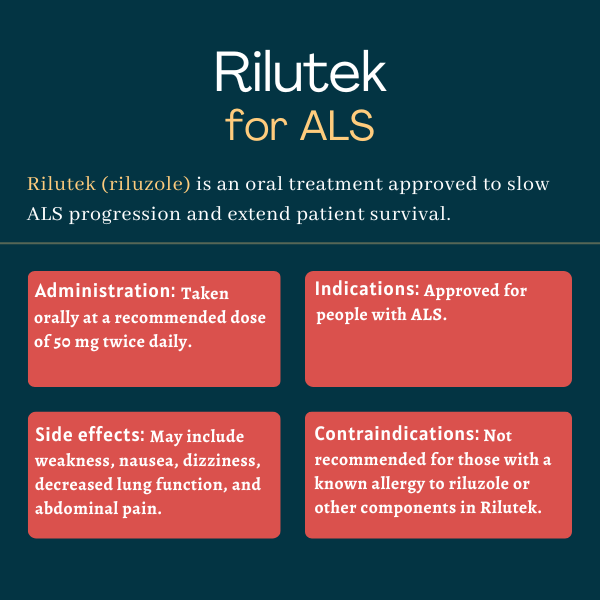
The U.S. Food and Drug Administration (FDA) approved Rilutek for the treatment of amyotrophic lateral sclerosis in 1995. Rilutek was the first medication to ever be approved in the country for the disease, but generics and other formulations of its active ingredient are now widely available.
Rilutek is an oral medication that slows the progression of amyotrophic lateral sclerosis (ALS) and the accumulation of symptoms by preventing nerve cell overactivation. The medication has been shown in clinical trials to delay the need for ventilatory support and extend survival in people with ALS. However, it generally cannot reverse symptoms that have already accrued.
In one of the trials supporting Rilutek’s approval, a significant reduction in the risk of death or need for tracheostomy, a procedure in which a tube is inserted through the neck to help with breathing, was observed in the first year of treatment. However, amyotrophic lateral sclerosis has a widely variable disease course and not all patients will respond similarly to a given therapy, so it is difficult to say when someone will start to experience benefits from Rilutek.
There is no known interaction between alcohol and Rilutek. However, patients on this medication should avoid drinking excessive amounts of alcohol because it’s not known if alcohol may increase the risk of liver damage while on treatment. It is recommended that patients talk with their healthcare team to discuss whether alcohol consumption is safe in their case.
There is no cure to date for amyotrophic lateral sclerosis. Rilutek and other available treatments for the disease also cannot reverse neuronal death after it occurs, but they can reduce the rate of disease progression and extend survival in patients.
Related Articles
 Fact-checked by
Fact-checked by