FDA Declines to Consider Approving NurOwn to Treat ALS
BrainStorm, agency likely to meet over possible next steps for cell-based therapy
Written by |

The U.S. Food and Drug Administration (FDA) notified Brainstorm Cell Therapeutics that it will not accept for review a company application asking that its cell-based therapy NurOwn be approved as a treatment for amyotrophic lateral sclerosis (ALS).
The FDA decision, in the form of a refusal to file letter, also indicated that Brainstorm may request a meeting to discuss the letter’s contents. Brainstorm had announced plans to submit a biologics license application (BLA) to the agency seeking NurOwn’s approval.
“While we are disappointed that the FDA has not accepted our BLA for NurOwn in ALS, we remain committed to NurOwn’s advancement as a treatment for this devastating disease. The company intends to request a Type A meeting and looks forward to continued discussions with the FDA,” Chaim Lebovits, CEO of Brainstorm, said in a company press release.
Phase 3 trial of NurOwn in fast-progressing ALS failed to show significant gains
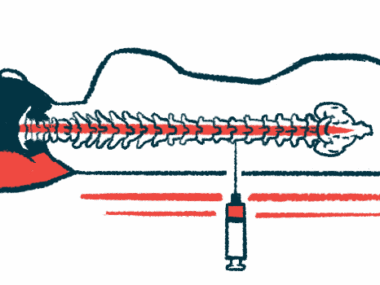
NurOwn treatment involves harvesting mesenchymal stem cells (MSCs) from a patient’s bone marrow, then maturing these cells in a laboratory so they secrete high levels of neurotrophic factors — signaling molecules that promote the health and growth of nerve cells. The modified MSCs are then injected into the patient’s spinal canal.
It’s thought this treatment could help slow ALS progression by promoting nervous system repair.
BrainStorm planned to support its BLA with data from a Phase 3 trial (NCT03280056) that tested NurOwn against a placebo in 189 people with rapidly progressing ALS.
The study’s main goal was to assess if more patients responded to NurOwn than a placebo. Responders were defined as those with a slower decline in the ALS Functional Rating Scale-Revised (ALSFRS-R), indicating a slower rate of disease progression. More precisely, patients had to experience an improvement of at least 1.25 points in their monthly decline compared with their rate of progression before treatment.
Top-line trial results, announced in 2020, did not meet this goal. The proportion of patients with a clinically meaningful slowing of ALSFRS-R decline did not significantly differ between those on NurOwn and those on placebo (32.6% vs 27.7%).
These findings led the FDA to determine trial data did not provide sufficient clinical evidence to support NurOwn’s approval, while also noting a modest excess of deaths among treated individuals.
According to BrainStorm, the lack of significance in the top-line data is probably attributable to this clinical trial having included a large proportion of patients with very advanced disease. About a third of enrolled patients, the company said, had a score of 0 on at least one subscale of the ALSFRS-R at the trial’s start — meaning it would have been impossible for them to show a decrease in these scores over time because of what’s called a “floor effect.”
After the trial concluded and all data had been collected and analyzed, researchers conducted additional analyses that excluded patients with scores likely to cause a floor effect. These analyses suggested that the ALSFRS-R declined more slowly in NurOwn-treated patients than in those given a placebo.
‘There were participants with beneficial clinical effects and overall changes in relevant biomarkers.’
Biomarker data also suggest that NurOwn had anti-inflammatory and nerve-protecting effects in patients given the treatment; effects not seen in people on a placebo, the company has reported. Notably, these benefits also were observed in patients with more advance disease — those removed from later analyses due to a floor effect — suggesting the therapy may slow disease progression regardless of disease severity.
In a joint statement, the Phase 3 study’s three co-principle investigators — Robert Brown, MD, with the University of Massachusetts Medical School; Merit Cudkowicz, MD, of Massachusetts General Hospital; and Tony Windebank, MD, with the Mayo Clinic — said they “support continued discussions with the FDA on the best path forward.”
“While the pre-specified primary outcome measure was not met, there were participants with beneficial clinical effects and overall changes in relevant biomarkers of drug effect. Understanding whether there are people with ALS who might respond better to NurOwn is important given the unmet therapeutic need,” the researchers said.